The modern life styles are the root cause of so many diseases that constitute a major share of health problems to us. In order to have a better understanding about the responsibilities involved in the health care it is necessary to analyse the current health care policies and practices instituted to counter the challenges. Holding individuals against their required choice would prove problematic as most of such policies are clouded in controversies (Cappelen and Norheim, 2005). The origination of issues like these are a continuing process in health care as the consumers are becoming more educated in the matter of patient rights as well as inherent legal rights. The future strategies and policies in health care have to be conceived so that the freedom of the patient either to accept or refuse a system of treatment and care is not at all jeopardized. Therefore, it is mandatory that informed consent in the form of voluntary agreement by patients having enough mental composure is necessary in delivering a health care system to them (Tess, 1997).
Informed consent is the procedure through which patients are able to make choices about the manner in which they undergo healthcare. The term has its origin from the ethical and legal rights the patients enjoy in deciding what can happen to their body as well as from the ethical duties of physicians while involving the patients in their healthcare. Informed consent is thus referred to as the permission that is obtained from patients to carry out a particular test or procedure on them. It is legally now established that informed consent is necessary before a patient undergoes any kind of research or medical treatment and that the same should be furnished in a language that is understandable by patients, one of which must be signed and dated by the patient with a witness. The document includes clear and rational statements that specifically describe the tests and procedures to be carried out and a statement that provides for the treatment to be stopped in case the patients desire so. As stated it is a legally bound voluntary requirement obtained in advance from the patients while they are fully conscious before initiating a treatment procedure including sterilisation and therapeutic abortions. The individuals from whom informed consent is sought must satisfy the minimum legal age prescribed for giving such consents. As per the state legislations in U.S.A, parental consent is required for medical treatment of the minors under the age of 18 as it is conceived that such minors lack proper understanding in taking decisions on their medical treatment (Melton, 1983). However, the legal age of 18 is arbitrary considering the fact that some minors are competent to make decisions of their own, and as such the legislations are formulated to protect the interest of the minors too to avoid any consequences of bad decisions made by elders (Kuther, 2003).
The provision of informed consent acts as a mechanism to favour patient autonomy and the same has been endorsed through several legal decisions based on the right to privacy of individual while it serves as the guiding principle in taking decisions in health care. Informed consent doctrine is thus designed to include three elements so as to validate decisions on medical treatment. These three elements are (i) the decisions made should be knowledgeable (ii) the decisions must be voluntary (the patient needs to exercise his/her free will), and (ii) and any decisions made by the patients are directly connected with their competency. The responsibility to fix the first element relating to the informed consent rests with the treating medical professional. This means the treating clinicians have to give the patients reasonable information about the risks and benefits of the intended treatment and that of other treatment alternatives. The second element namely, voluntariness is the decision making process of the patient without any coercion from others. The third element of informed consent constitutes competency of the patient which is identified as the most important aspect of informed consent and though it is the burden of the patient to exercise his or her competency, it is a must that the same is satisfactory to the researcher or the clinicians. The law stipulates that adult have the required competency to make decisions of their own until and unless proven otherwise. Therefore, in order to validate discussions on ethical and legal principles, the legal theories and clinical decisions are to be dealt considering they are two separate entities (Rosenfeld, 2002).
History and Progress of Informed Consent: The development of patient autonomy and changes in medical practice
The basic principle of informed consent which is targeted against the lawlessness of health assistance reflects the autonomy concept and the auto-determination of the person who wants medical attention. This has prime importance in the evolvement of doctrinal approaches and legal interpretations influencing the day to day activities of medical care. The concept was originated and developed through the years and was conditioned by the religious and moral dictums and moral aspects necessitating new treatment methods and biotechnical applications and procedures. The principle of informed consent has its beginning from the times of Greek and Roman civilizations wherein the physician’s acceptance was subjected to the patient’s approval on the basis of the information provided and the quality and social position of the patient. However, the present concept of informed consent in health care practice and research was developed during the mid of the twentieth century which was more or less a reaction to the abuses rampant in the rendering of medical care. It prompted the implementation of ethical principles in every aspect of medical research and care. There were disputes among the historians on the amount of informed consent that was put in medical practice prior to the above period. Most of them argue that the physicians of those times disregarded patient’s rights and views while a few others are of the opinion that there were high respect for the knowledge and autonomy of the patients. But it is evident that the doctrine of informed consent has acquired more attention in the last few decades in the medical practice in Europe and USA (Mallardi, 2005).
In research, the doctrine of informed consent began in the USA in the year 1930 with the courts’ intervention in the issue and subsequently upholding the requirement of knowledgeable consent in all sorts of medical research. The creation of ethical codes including informed consent was initiated by the judicial courts in retaliation to the cruelty done by the Nazi regime while they conducted experiments with the human subjects. It is relevant to note here that the Nuremberg Code asserted that in every research the elements of voluntary, competent and informed consent were necessary, though it was exercised only within the ethics of researches. Nevertheless, the abuses in the medical researches continued till the year 1960 when there were efforts to explain and codify the principles of informed consent. Thereafter in 1964, the World Medical Association insisted vide the Declaration of Helsinki that informed consent was necessary for every research for the conduct of investigation. Subsequently in the year 1966 Henry Beecher wrote in the New England Journal of Medicine placing 22 cases of unethical practices he has noticed in the conduct medical research. In the same year the US Food & Drug Administration stipulated specifically the need for informed consent consequent to the Jewish Chronic Disease Hospital scandal. This was followed by the creation of a policy by the US Surgeon General asking those who have received funds for research to submit their studies for institutional review to confirm whether they have adhered the principles of informed consent (Wood et al., 2001).
In spite of all these developments the abuse continued. In 1972, the news about Tuskegee syphilis experiment caught the medical world unawares. It was revealed that syphilis experiments were in progress on hundreds of African-American men for 40 years without revealing the information about the study and the treatment was withheld during the period. The experiment caused death to most of them due to the disease. Their partners were also infected and their children born with the disease. This tragic incident forced the medical research world to form the US Federal advisory committee called ‘the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research’ which submitted the Belmont Report demanding the implementation of medical ethics doctrine as mandatory for any kind of medical research. According to (Wood et al., 2001) the report released from 1974 to 1978 has stipulated that the researchers should adhere to the three elements that constitute the informed consent viz,
- “Respect for persons—individuals should be treated as autonomous agents, and persons with diminished autonomy are entitled to protection” (Wood et al., 2001)
- “Beneficence—possible benefits should be maximized and possible harms minimized” (Wood et al., 2001), and
- “Justice—those who realize the benefits of research should share in its burdens” (Wood et al., 2001).
After the submission of the above report “Common Rule” was proposed in the year 1986. Prior to this and based on the 1981 policy founded by the Department of Health & Human Services (DHHS) the regulation was made applicable to every government agency in the US. It was codified in the year 1991 and the system of Common Rule was placed as the regulatory for human research. Thus the recent history about the institution of informed consent is basically dominated by the developments and events that occurred in the United States of America. However, many researchers have opined that the present principle of first-person informed consent which came into being as an offspring of the civilian rights in the States cannot be made applicable elsewhere considering their respective socio-economic and cultural backgrounds where the medical conditions are totally different. If this doctrine is to be applied there it would be termed as imposition of ethical imperialism. This school of thought was appeared in 1992 in The New England Journal of Medicine. The authors of the paper namely, Carel IJsselmuiden and Ruth Faden (Ijsselmuiden and Faden, 1992) have supported their claims regarding non application of the doctrine in Africa by placing forward the following three arguments. According to Ijsselmuiden and Faden (1992),
- “It is culturally inappropriate because individuals are not autonomous;
- The subjects are not “competent” or the communication difficulties are insurmountable; and
- Urgency makes informed consent requirements unreasonable (i.e., informed consent slows down the search for solutions to urgent health problems)” (Ijsselmuiden and Faden, 1992).
They were of the opinion that because of the cultural difference observed during the anthropological research meant that informed consent was not at all applicable in the African continent. They argued that African culture was static and homogeneous and urbanization was out of question for them. But later on they rejected this view for the reason that such an argument would challenge the cognitive capacity of the African adult individuals and accepted the fact that the meaningful informed consent is applicable in Africa too (Wood et al., 2001). Subsequent to this, Karim et al. (1998) have evaluated the procedure of informed consent in the matter of HIV testing conducted in antenatal clinic in South Africa. Their study was published in the American Journal of Public Health in the year 1998. In this connection Karim et al. (1998) state:
Although consent was sought and women were consistently told that their participation was voluntary, 84 percent felt that it was ‘compulsory to participate’. A large majority said they understood that they were ‘free to quit the study at any time.
It was found that about one-third of the women population who were subjected to the study viewed that the care they were given would cease if they stop participating in the study. Therefore, the researchers reached a conclusion that though the consent was informed it was not at all voluntary. It was evident that the cultural obstacles still remain in the matter of implementing the concept of informed consent among the African individuals. However, there are sincere efforts by the Medical community to overcome these barriers particularly in the matter of researches conducted on vulnerable people who have no access to modern resources to counter the impact of fatal diseases. After all, the first principle envisaged in the Nuremberg Code which insists that comprehending consent, competent and informed is absolutely essential stills stands valid as a guide to research ethics (Wood et al., 2001).
Recent Developments in Medical practice
The recent trends in the health care have already highlighted the necessity to continual assessment of the principles of medical ethics inclusive of informed consent.
It has become legally viable and is focused mainly on preventing any law suits against the medical institutions in USA rather than favouring the individuals who are partaking in the research of medical therapy. Many of the forms are now more complex and do not extend the concept of meaningful comprehension and voluntary consent. This happens in the cases of service providers and researchers as well as manufacturers for fear of litigation against their service or products, jeopardizing the very concept and intention of the informed consent. Most of the participants of the research study or clinical therapy do no pay any attention to these intricate forms as they have a pre notion that the consent given will only protect the institutions and not at all the interest of the individuals. A section of the researchers were on the move to revise the forms to safeguard the interests of the participants so that proper support could be provided to them to assess the idea of comprehension. At international level informed concept is threatened by the frequent discussions originated due to the HIV/AIDS epidemics (Wood et al., 2001).
Informed consent has been structured to provide for the patient’s right to autonomy and relates to the document based explanations, and other rights of the patient prevalent in healthcare sector. The concept originated in the West, and the significance of informed consent, has been now implemented across the world, specifically in context of the proclamation of the patient’s bill of rights which was introduced in Korea in 1986. However, there are considerable shortcomings in the present research of informed consent as it is actually framed and dealt with in clinical settings. Complications arise in the context of discrepancies in terms of perceptions of patients’ families and healthcare professionals regarding the specific process related to matters that need urgent attention (Lee et al., 2008).
In terms of the objectives of informed consent, the most significant point is that the patients are allowed to utilize the opportunity to become informed participants while taking health care decisions about themselves. Mostly, it is accepted that informed concept in entirety implies a discussion with the patient in relation to the nature of decisions and procedures as well as reasonable alternatives which are applicable in terms of the required intervention. It also includes mention of relevant uncertainties, benefits and risks in the context of every available alternative. An important part of informed consent is that of making assessments of patient understanding and the acceptance of the given interventions by the patient (Lee et al., 2008)
For informed consent to become valid, the patients are supposed to meet the given conditions so that they are legally competent to make the decisions. Informed consent has to be voluntary because it is prone to coercive circumstances that surface in medical treatment. The patients, in generic, feel vulnerable and powerless in such situations. In order to encourage the sense of involuntary decision-making, physicians are required to testimony before the patients that they are participating in decisions and not just signing a simple document. Given this understanding the process of informed consent is viewed as an invitation to patients to take part in their health care decisions. The physician also has an obligation to make appropriate recommendations and to share his or her opinions with the patient in regard to the given procedures. It is also important for the patient to comprehend the given information which requires the discussion to be conducted in a manner that a normal individual understands the terms and conditions and can make proper assessments along the way. Basically informed consent allows patients to know what they like to do by asking them whether the given procedures are acceptable to them. For instance, informed consent is considered to be appropriate when blood is drawn from patients. This sort of decisions that require basic information entail a process needing low levels of patient involvement in view of the high levels of community consensus (Lee et al., 2008)
Recent developments in medical research have raised a number of issues about the efficiency and effectiveness of government regulations governing the manner in which medical research is conducted in all aspects of clinical trials and surgical and medical treatments and research. The legal definitions of human subject and private information that have been regulating the course and practices adopted by research for the last two decades are currently being analyzed in view of the ambiguity that arises due to the family research in medical and human studies. Researchers are known to call for information connected to family history in order to track and characterize genes and cells of patients belonging to larger families. Piling up such information mostly requires researchers to ask patients and subjects about the health conditions of collateral family members.
Issues will arise if these family members are to be treated as subjects for the purpose of providing informed consent. Such problems have previously led to considerable debate and a large scale exercise to re-examine the present federal regulations and rules regarding informed consent. Future practices and regulation in the context of informed consent should make a review of the developments made in the present federal regulations and the extent to which they provide to resolve the existing issues in medical research. Secondly, a review should be made of the usual practices in medical research as it will further the context of regulation and guidelines for addressing the given issues in medical research. Thirdly, it is also required that all new regulations and guidelines should contain measures for protection of individuals and their family members who take part in the research exercises while giving support to related activities in medical research.
In the case of biomedical research which is done with the participation of human beings involves four elements of ethical concerns connected with values such as dignity, integrity, autonomy, and overall privacy. These elements have acquired specific legality in USA and warrant safety of the research participants. The same legal protection is extended to the informed consent and retention of privacy also. The main principle underlying this legal provision is that the use of humans in biomedical research is solely allowed for the purpose of beneficence and non-maleficence. Moreover, it will sanctify the trust in participant relationship and enhance personal dignity while adhering to the necessity of autonomy of the participants in taking voluntary decisions. It also denotes competency in decision making and confidentiality of the personal information given (Kapp, 2006 & Veatch, 1987). The historical as well as philosophical background that led to the prevailing system of governmental control has culminated in the publication of American regulations by the Department of Health & Human Services (DHHS) in the year 1981 which was purely based on the recommendations placed by the Belmont Commission instituted by the National Research Act 1974. Most of the medical institutions functioning in USA have adopted these regulations for application in their research protocols. Researches conducted in the testing of drugs and medical devices are now regulated by the Food & Drug Administration but the Common Rule and informed consent requirements overlap the regulations considerably (Kapp, 2006).
Whatever be the regulations, the act of obtaining informed consent from potential research subjects is obviously not a straightforward procedure. Researchers are required to concentrate on a wide range of matters while providing information to research participants and getting their informed consent. Such issues are inclusive of the styles, formats and timing of information and the kind of consent. There is also a strong need to be keen on the context of the level of consent and to see whether they should be inclusive of something that is more than just consent for collecting data only. The environment in which the research is carried out and the ethical orientation of the studies have a strong impact on the approach of researchers in such issues. But the decision about informed consent is largely driven by regulatory, ethical and legal frameworks in which the research is carried out. A number of changes have taken place in terms of ethical regulation in the context of medical and social research that have occurred in response to the moral, political, legal and institutional influences, which means that such issues are likely to be subjected for more modifications in the days to come.
Aims and Objectives
The doctrine of ethics in informed consent is founded on the need to promote the values of personal well being and self decision making in health care. In order to ensure the above values the patients who have enough capacity to make decision of their own should be allowed to exercise it voluntarily. While doing so, they must be equipped with all relevant information relating to their condition of illness and also the risks and benefits of the treatment together with the options available and the consequences thereon. The patients must be informed about the uncertainties that might accompany any treatment and service taken or on the information provided. Since informed consent is mandatory and binding on both the parties it is shrouded with a number of implications. Though it has legal validity it is more or less an ethical entity. Informed consent is rooted through the legal presumption that the adults who are capable of making decisions can either reject or accept any kind of medical interventions voluntarily and without any coercion from outside elements, on the basis of their own choices with regard to their personal goals and values. But in reality this voluntary acceptance or rejection of a medical service by an adult is not at all absolute. Moreover, the ethical doctrines simply allow the service provider to withhold certain information from the patients when the physician is satisfied that such information might aggravate the condition of the patient badly. Recent changes in medical care and societal outlook in the American life have necessitated the reexamination of patient-physician relationship which reflects at all levels of understanding about the legal rights and ethics involved in the disclosure of information and informed consent between the patient and medical practitioners (Jonsen et al, 1998). Therefore, the present study is focused on the issues relating to the ethical and legal implications of informed consent which are applied in the current health care system. The researcher’s aim is to find answers to the following research questions:
- What are the ethical and legal implications of informed consent in healthcare?
- Will the present informed consent justify the elements of voluntary, competency and confidentially in patient-physician relationship?
The answers to these questions will give an insight to the pros and cons of the ethical and legal aspects of informed consent. Moreover, it will help to find out new measures to allow more confidentiality to the information provided by an individual in informed consent while availing legal rights to protection of one’s privacy.
The main objectives of this research are:
- To conduct secondary research to establish the present ethics and legal rights inherent in informed consent
- To examine the contributions of healthcare institutions in observing the well being and privacy of the patients.
- To identify the potential problems in informed consent and to find solution to the problems to safeguard the interest of the patients at both the ethical and legal levels
- To find out whether the participants/patients are aware of the status, function, and remit of written consent
In order to find out reasonable answers to the above research questions and objectives the ethical and legal constituents of informed consent are placed under the following different chapters.
- Introduction: It gives a brief account of background, problems and leads to the research approach.
- Literature Review: The entire data collected is reviewed here.
- Methodology: The data collection methods and analysis are done under this head
- Results (Analysis): Findings derived out of the study are elaborated in this chapter.
- Discussion: The findings are analysed and discussed here.
- Conclusion & Recommendation: The outcome of the research and recommendation for furtherance of the study are given under this chapter.
An Ethical Model of Informed Consent
Researchers have used several approaches in the context of ethics relating to informed consent. Social research ethics is directly related to medical research ethics. Structures of tactical medical research has been devised after it was found that there has been considerable abuse experienced by subjects of human research after the Second World War. Provisions were made in this context in the Nuremberg Code and the Helsinki Declaration. In 1968, local research ethics committees were created in the United Kingdom while the USA also had similar functions that were supported by the federal law for the purpose of regulating medical research and ensuring compliance with the given framework. But there is considerable evidence to the effect that research in social and medical science continues to be conducted in a manner that does not respect the rights of individuals. There have been several scandals in many developed countries where the organs of dead children were kept for the purpose of research without the consent of parents. Such issues highlighted the manner in which unpractical research practices have been continuing (Indian Council of Medical Research, 2004).
In view of such conditions, the department of health developed the research governance framework that brought together different statutes and guidelines for providing an ethical and coherent environment in health care research. It has been found that in health and medical related research studies, rights and principles based upon the deontological methods appear to be used whereby ethical decision is made considering the consequence and outcome of participation in research activities related to the rights of an individual or on the basis of moral codes of conduct. Principal-based approaches have been warned the compliance with moral principles while rights based approaches have been involved with issues such as respect for people and protecting them from harm and participation in research activities (Jinadu et al., 2007). Some of the moral principles considered by researchers are:
- People must be allowed to practice autonomy whereby they are free to take their own decision in the matter of participating in medical research.
- Non-maleficence implies that medical research activities should not bring harm to any individual.
- Beneficence implies that medical research should create benefits for people.
- Justice comprised of a strong informed consent so that people are treated on equal terms in the medical research process.
Many social researches and even arguments have proved that these methodologies do not actually bring benefits for social research in view of the ethical dilemma that arises in social research activities. Moreover, social researchers complying with the given ethical regulations in regard to research can impact the issues that are being researched, whereby it becomes impossible to get the desired outcomes from the research. Such issues are specifically relevant in experiments relating to psychology but are also relevant for research activities in anthropology, sociology and ethnographic research studies. There are considerable debates about the relevance of ethical decision-making in social research activities because they include commitments in the context of individual’s rights in terms of protection of privacy. This also implies respect for participants and respect for social science while promoting and providing knowledge (World Medical Association, 2003).
It is usually expected of social researchers to justify themselves and their colleagues about complying with the given ethical procedures. However, some researchers have held that such procedures used mainly in complying with systems of self-regulation as the membership to such organizations is voluntary and the instructions are not forced legally. Moreover, the guidelines are made vague intentionally, thus allowing researchers to integrate them without interpreting in order to meet the needs of the given research they have undertaken for their own specific orientations related to research ethics. Such patterns have permitted social researchers to make use of situational relativist approaches whereby ethical decisions continue to be made on the strength of the moral stand taken by researchers on the basis of matters relating to specific research projects (Jones, 2003).
At the same time, after the Economic and Social Research Council (ESRC) created the Research Ethics Framework, many research organizations and universities have started organizing themselves by ensuring that research activities relating to human subjects become focused to mandated ethical reviews that will inevitably have considerable influence upon the liberty available to researchers in making their own decisions in the context of ethical matters (Kuczewski et al., 2004).
Autonomy and how it affects the medical consent process and medical practice
Informed consent is a topic that is widely debated upon these days. The main purpose of getting informed consent is to verify that the patients have been duly informed about the treatment options that are available to them and also the risks involved. The patients have to understand the options carefully and then choose the one that is best for them. Instead of protecting the patient’s right to autonomy it is now seen that informed consent has become a form of evidence that is protecting physicians from all kinds of medical malpractices (Rainbow, 2002). Medical informed consent will allow the physician to diagnose and treat the patients and at the same time the patients have the right due to medical informed consent to accept or reject any kind of clinical evaluation and treatment or both. With the help of the medical informed consent, the patient and the physician together are able to exchange ideas that improve their relationship (Paterick et al., 2008).
It is well known that the autonomy of patients comprises an important part of informed consent and it is the duty of medical practitioners to keep patients informed about the medical procedures. A more pertinent issue also relates to patient comprehension about the given information because without it they are unable to achieve actual autonomy while making decisions about their health care. If it is ensured that all aspects of informed consent are complied with there will be less number of malpractice claims and higher level of patient satisfaction in addition to a better image of medical professionals. However, nurses are required to become more aware of the state laws in the context of their practice, which also includes actions of nursing practices (Hyder et al., 2006).
Today physicians tend to attend to as many patients they can in a day as they wish to earn more money and that is the reason why they try to get the informed consent from the patient as quickly as possible so that they can perform the treatment and move to the next patient. Physicians want to save time and they offer few options to the patients and give very little explanations to them. The patient does not even get to understand all the available options before him and is left confused most of the time. Sometimes they are pressurized by the physicians to sign the consent form quickly and due to that they are unable to make mature decisions regarding their treatment. This results in patient dissatisfaction with their physicians and treatments (Rainbow, 2002).
In other words, the consent process can lay the foundation on which the relationship between a patient and a physician can be built. Today the physicians will have to realize that informed medical consent is an important educational process and it can potentially affect the alliance that exists between the patient and the physician and work for their mutual benefit. The patients will have to be given equality by the physicians and they should be educated so that they are capable of making the informed choices. It has been observed that when the medical informed consent is taken seriously by the physician as well as the patient, the relationship between the two will emerge as a true partnership. It is only then that decision-making authority and responsibility for outcomes are shared by them (Paterick et al., 2008). The topic of informed consent has emerged as a prominent area in view of the constant changes that have been taking place in the governance of research in the US and other developed countries. Medical research is now considerably regulated in terms of the frameworks in which social researchers have been working (Tinker and Coomber, 2004).
Beneficence and how it affects the informed consent process and medical practice
Autonomy and beneficence work efficiently in the relationships between patients and physicians under conditions when mutual approaches to treatment are agreed-upon, because the only components of autonomy pertains to the fact that all patients exercise freedom of choice. Making use of undue influence or coercion nullifies the true meaning of autonomy. Another factor responsible for creating the feeling of incapability of patients in the past in the matter of making effective decisions about their health care was the lack of adequate knowledge. Most patients gave consent for surgical processes as they were not able to understand them, which in turn brings in enhanced legal claims about undisclosed risk and the consequent outcomes. There has been considerable increase in the number of malpractice litigations for the consequences of claims arising due to misrepresentation in surgical procedures (Berg et al., 2003).
Informed consent is crucial in medical practice as physicians can use it as a form of evidence during treating the patients. The consent of the patient has to be presumed instead of being obtained in emergency conditions when the patient is not conscious and the process is not completed due to the absence of surrogate decision-makers. Mostly, the presence of the patient in the hospital board, clinic or intensive care unit does not imply that consent is given in the context of all procedures and treatments. The values and wishes of the patient could be at variance as compared to the physician’s values. Although the principles of respect for patients requires medical practitioners to do their best by including patients in the healthcare decisions, the principles of beneficence demand that medical practitioners have to often take decisions on behalf of patients when their life is at stake (Berg et al, 2003).
In recent times, considerable efforts have been made to improve the physician-patient relationship. It has brought in significant changes as it has replaced the earlier quiet world of doctor and patient with a more educated and informed patient participation in medical treatment. Patients are involved in decision-making and timely decision on the part of the physician can save the life of the patient. The decisions at times have to be taken immediately as it may be an emergency situation and the patient is asked to fill the consent form. In this context, consent is understood as voluntary agreements by people that have appropriate mental capability in making intelligent choices about the actions that are proposed on them by another person. Such actions relate to the administration of medicines or performing specific medical procedures on patients (Lustig, 1996).
The concept of the ethics of patient autonomy has made sure that for all medical treatments or interventions there should be routine provision of informed consent. Issues relating to informed consent can significantly alter actions of nonconsensual to consensual actions. However, it is recognized that informed consent is not required for routine procedures such as angling touching and bumping that can occur during surgical procedures. Informed consent is also understood as the medical practitioner’s legal duty to provide all the information to his or her patient so that the patient is allowed to understand the process before giving consent for surgery. Informed consent also requires that there should be complete recognition of the risks, processes, and the probable outcomes by patients undergoing treatment (Lustig, 1996). According to Lavery (2003), the patient has a right to receive proper information irrespective of anxieties created by the given information or the extent of indecisiveness of the patient. At the same time, the complete explanations can be modified if apprehension of the patient has potential to cause adversities in the context of his or her health condition. This theory is referred to as therapeutic privileges and relies mainly on the beneficence model relating to the protection of patients’ interests. Beneficence provides for guidance on ethical issues to physicians and nurses and also includes the principles relating to doing no harm and not depriving other options such as people of their freedom. However there is a tendency to make excessive use of therapeutic privileges when physicians fail to obtain informed consent from patients (Lavery, 2003)
Justice and how it affects the informed consent process and medical practice
Physicians will have to look at informed medical consent from an ethical perspective that has been codified by statutory law in many states as well as from a layman’s common-law perspective that requires medical practice to be more consistent along with a good standard of care. It is crucial for the patient-physician relationship that both the partners understand and learn to accept a fair degree of autonomy in the entire decision-making process (Paterick et al., 2008).
According to Capron (2006), many courts in the United States have declared people as incomplete and for making decisions on the basis of the patient’s ability to manage his or her own property to communicate meaningful choices. In general, the incomplete conditions exist in the case of mental retardation, mental illness, physical incapacity and excessive use of drugs and alcohol (Druml, 2004). In most cases courts and patients choose the closest relative of the patient for acting as proxy while making decisions for medical procedures. Family members are known to have knowledge about the patient’s preferences in relation to medical care. But the nurse has to be fully committed in giving respect to the rights of patients while recognizing difficulties faced by the surrogate decision makers during the choice making process. The duty of securing assigned informed consent is not entirely belong to the nurse. Many times the authorities from healthcare organizations insist that procedures be performed after executing the written informed consent. Such documents are inclusive of witnessing the signing of the consent form by the patient. It is known that the surgeon in the hospital is more knowledgeable than the nurse in the procedures that have to be performed and is hence the best choice in terms of capability to inform patients about given procedures (Brody, 2004).
Virtue and how it affects informed consents/ Balancing moral principles and rights
The concept of informed consent signifies that every individual must have the basic right to make decisions that can affect his or her health. This would mean that the patients will have to consider the risks that are involved and also the benefits they will get by making those medical decisions. In view of the fast changing nature of medical research, a large number of literatures have been published about informed consent during the period 1998 to 2004. But these literatures have included books and papers that were published before this period and believed to have made a significant contribution to debates and issues relating to informed consent. There is a strong need to focus on informed consent related qualitative research that comprises of conventional methods of data collection, such as observations and interviews (Paterick et al., 2008).
Collste (2007) opined that in most cases hospital administrators are not held responsible by courts unless there is evidence that the medical practitioner has been repeatedly performing procedures without obtaining consent from the patient. Once informed consent has been obtained, the patient has a right to withdraw it till the time the procedure has not been commenced. The patient can give any reason for this choice and has to sign a written form which is then included in his or her medical chart so that the medical practitioner, nurse and the institution can be protected from future liabilities if the patient blames them for not providing proper treatment. A note is required to include names of witnesses in front of whom the patient signed the withdrawal request. Additionally, the nurse has to record all comments made by the patient, his or her emotional state and whether the patient was in a coherent state in terms of being free from the impact of any medication.
Healthcare professionals and patient relationship
When patients refuse to undergo surgery or other forms of treatment, healthcare professionals have to rusticate seasons for the same. Refusal by patients can result in strained relationship between the patient and doctor. In circumstances when it involves a number of added questions that can arise in the informed consent. If they are above 18 years of age and married or are financially independent, parent’s involvement is not considered necessary. At the same time there are exceptions in the case of emergency situations or when the patient is pregnant or suffering from sexually transmitted diseases or is dependent on drugs. The parents of patients and hospital representatives had to refer to court decisions for overturning refusal of treatment by patients because it could pose a serious threat to their health and lives (Dworkin, 2005).
Health care professionals and parents are required to account for different aspects of shared responsibility while making decisions involving minor patients. As far as possible, healthcare professionals have to collect the informed consent of parents as well as the consent of the child patient. Such processes involve assisting the child in understanding the nature of his or her medical condition, telling the child about what would happen during the procedures and soliciting expressions of consent from the child so that he or she accepts the surgery or other modes of treatment. The significance of such concept of complete disclosure in the context of medical treatment and surgical procedures has been analyzed on a continuous basis by medical researchers and analysts. Knowledge of the past historic events relating to informed consent has been of immense help to healthcare professionals in understanding meaningful approaches (Dworkin, 2005).
Research conducted by Fisher (2006) in the area of informed consent has been the consequence of the ontological theories and the resulting ethics in the matter. The ontological theories are generally accepted to have their origin in biblical times and are considerably related to the Ten Commandments in being reflective of every individual’s necessary duties towards society (Atkison, 2004). Consequential ethics draws its ideas on the belief that the end justifies the means and the acceptance of principles pertaining to utilitarian ethics developed from these two concepts. This principle also implies creating maximum benefits for the largest number of people. However, it is known that ethics implies a number of other principles because of which autonomy is usually understood as the most significant element in informed consent. Autonomy is also reflective of personal freedom for taking specific actions group within the prevailing restrictions and is therefore more of a core concept in relation to informed consent.
According to Appelbaum et al. (2002) a number of court cases provided for and identified as medical practitioners’ duties for providing patients with specific and detailed information about the treatment that they have to undergo. In cases where patients underwent medical procedures and suffered disabilities of which the courts were not successful in identifying the extent of disclosure, it was necessary to remove the shortcomings. The understanding of patients about the surgical procedures along with the related risks and advantages were major components in several legal cases in the past. Landmark cases proved that informed consent comprised of disclosing the amount of risk would impact upon the decision of a reasonable individual. The courts also allowed the medical fraternity to determine the incidence of injuries and the extent of possible harm including alternative choices of treatment and the possibilities of this inability or the death. Such information was considered as patient oriented standards of disclosure which became the precedents in several courts of the US (Freemon, 2004).
Baker (2006) has confirmed that the legal theory relating to informed consent represents different aspects of tort law, which provides protection to people from harm to reputation, property and body. Moreover, this law has also enabled compensation for injuries and has prevented corrupt practices and punished people who have been involved in wrong behaviors. Assault and battery are considered to be the main issues of intentional tort. Assault is a compulsive threat for doing physical harm to another individual in addition to the ability possessed to inflict such harm. Battery is the intentional harming of an individual without his specific consent. In any medical situation when surgical consent is not obtained, the medical practitioner can be charged with the offence of committing assault or battery. Negligence is also a part of the theory of tort law pertaining to informed consent. Negligence in this context implies failures for providing adequate care to avoid inflicting harm on the patient. A medical practitioner’s duty to make use of appropriate share arises from well established practices of relationships between nurse and patient as well as physician and patient. Such standards of caring specifically provide for the expected conduct of individuals in given situations. This conduct is determined by way of the conduct of a prudent person under similar circumstances.
If a medical practitioner is imposed of charges for having failed to give the required information to the patient, the plaintiff has to prove that the medical practitioner did not comply with the norms of the applicable industry standards pertaining to healthcare. Standard of care in such cases is related to information that any reasonable medical practitioner is required to disclose under such circumstances. Industry standards relate to national standards for patient that is presently practiced in terms of community standards pertaining to local practices. The plaintiff is also required to establish what a normal patient should have decided in the same situation and that lack of information led to the given condition or injury. Most states in the United States are supportive of this objective test although a few states endorse subjective tests more often (Benedetto, 2005).
However, there can be exceptions that apply in terms of full disclosure of information by physicians when the risk is adequately known and when the patient is agreeable to undergo surgery irrespective of the given risks. Such exceptions also apply when the patient voluntarily agrees not to be informed and consent is not possible when disclosure prevents the patient outcomes. For instance, in the case titled Canterbury versus Spencer, a 20-year-old man had undergone back surgery without being given information about the risk of analysis. A few hours after the medical procedure, he fell off his hospital bed and was left unattended for a few hours because of which he was paralyzed and suffered from breathing problems. As a result, he needed additional surgery that is allowed in restricted muscle controls. The court gave the ruling that the surgeon was duty-bound to provide specific information to the patient before starting treatment as such information could impact on any reasonable individual’s decision whether he should go for surgery or not (Harvey, 2003).
Informed consent is accepted as evidence that the individual’s consent was made on the basis of his or her understanding in the context of the provided information. Although it is not easy to ascertain whether the patient has comprehended the given procedures, such practices can assist in eliminating unnecessary litigations in the future. There are several issues involved in this kind of understanding that includes the ability of the patient in the matter of language, hearing, intelligence, coherence and literacy (Beauchamp et al., 2001).
In another case titled Melissinos v Phamanivong, the medical practitioner did not obtain the required informed consent from a refugee who did not know English. The refugee’s thumb, middle finger and index finger were amputated due to an accident at the workplace. The medical practitioner took the help of interpreters and tried to obtain the patient’s consent for providing a Morrison process that required the removal of a part of the patient’s big toe for reconstructing his thumb. However, there was a direct refusal from the patient and after one week a different interpreter was spoken to and asked to explain the entire procedure to the refugee. Again, the surgeon did not succeed in informing the patient about the involved risks. Rather, the surgeon informed that after the process the patient would have a normal big toe following the surgical process and that the surgery was essential if he desired to continue his work. Eventually, the patient gave his consent for the procedure that was mentioned on the document as skin graft, drainage and incision following the surgical process. Subsequently, the patient suffered from infection and a part of the reconstructed thumb was lost. At the same time there was considerable development of deformity in the patient’s foot and hand, because of which he became considerably distressed and suicidal. After the court proceedings were conducted, the physician was held to be negligent in having the required consent. The surgeon had not disclosed the risks of infection and the possible consequences to the patient prior to the surgery and that the patient was made to give his consent under coercion (Boris and David, 2005).
Informed consent, in the context of readability also assumes significance in the comprehension and effectiveness of the patient. Most formats of informed consent are known to be very difficult for ordinary people to understand (Henderson, 2006). According to the US census, almost 15 million people living in urban areas do not have the ability to understand the English language effectively. Moreover, the health care system in the United States is known to make use of inadequate interpretation systems in healthcare. Healthcare facilities are known to make use of ad hoc interpreters such as clerks, employees, friends, family members and even strangers available in the waiting rooms. They are often called upon to explain the meaning and interpretation of medical instructions to patients. It is well established that such interpreters often commit serious substitution or omission errors that could result in changing the meaning to have an adverse impact on the decision of patients. Moreover, the patient’s family members or friends may not be able to explain all the details of the proposed medical intervention in the belief that the omission will be for the betterment of the patient (Health Research, 2006).
The circumstances relating to cultural and information disparities impact on the understanding of the patient because patients usually expect to receive information regarding the proposed surgical treatment passively. After being instructed before one-time readings they are made to sign the consent form without obtaining copies for themselves. The wordings in the consent form are usually quite technical in terms of medical care thereby decreasing the understanding level of the patients. Such circumstances increase the chances that the patients are inclined to take legal action against physicians (Institute of Medicine, 2005).
The patients will be in a better position to understand and then fill the consent form if it is written in a language they can easily conceive. The literature is clear about the fact that there are several problems related to informed consent which constantly keep appearing in the news. There appears to be conformity that informed consent is necessary for medical research as well as treatment but there are several problems inherent in it. In spite of the large number of issues regarding informed consent, the current literature is considered to be quite sparse and inadequate in providing researchers with concrete conclusions to carry out effective decision-making across different cultures and communities (Henderson, 2006).
Research papers have an interest in a wide range of issues relating to informed consent and most of them are quite provocative in addressing controversies invoked by publishers of research papers. Researchers have argued that citizens have moral duties to take part in medical research because research is considered to be good for people in terms of enjoying all the medical facilities that science has been able to provide to humanity. Hence, they must willingly take part in experimentation and give their consent to the required medical procedures. Further, people who do not take part in it are usually referred to as free riders, but they wish to enjoy the advantages of the sacrifices made by the past subjects without contributing their own efforts. Researchers have claimed that individuals must take part in research activities even if they are risky because in a society, citizens are expected to take risks for betterment of it. Proponents of medical research argue that if it is right for society to expect citizens to risk their life in fighting for their country, it is also right for the people to take risks while taking part in medical research. There are several debates about informed consent whereby researchers have paid immense attention to the rights of patients, while not much significance has been given to their responsibilities (Henderson, 2006).
Informed consent can be taken only if the information is understood fully by the patient. Under no circumstances should it be taken under any coercion as has been reported in many cases. The patient or the participant has to be educated first about the medical process and the options available. Cultural and information disparities become a hurdle in getting the informed consent from the patient. It has been argued by researchers that if individuals take part in research activities it eventually proves to be in the interest of the society. Instead of believing that opting to become subjects in medical research is a sacrifice, participation in such activities should be viewed as being beneficial to the society. It is mostly assumed that medical experimentation is risky as compared to traditional treatments. Many researchers argue that high standards of informed consent are necessary for research as compared to medical treatment. Most of the medical treatment is considered to be as risky as research that has been carried out on patients. Medical professionals who conduct experiments are obliged to comply with straight processes and have to be ready to take care of any complexities of side-effects that may arise due to such experiments. Prior to conducting human trials, medical professionals are required to have strong reasons that the given experiments can be of immense help with very less chances of harm being inflicted on subjects. On the other hand, many traditional treatment systems have not been entirely proved to be of much benefit and medical professionals have been found to avoid such problems. Hence, subjects in medical research are not required to be altruistic but are encouraged to volunteer because of their sense of responsibility towards society and their self-interest (Boris and David, 2005).
These efforts are not required to involve with the large number of complicated ethical issues in relation to the research that uses modern digital technology and visual processes. It also does not involve the matters relating to consent relative to quantitative research. It is thus important to explore problems of informed consent in qualitative social and medical research as it is carried out on vulnerable groups that include children, aged people and those suffer from physical and mental health complexities. Amongst such groups informed consent is understood as important while conducting research. It is therefore important to review the legal, ethical and regulatory perspectives of informed consent as well as putting the practice into operation (Boris and David, 2005).
There is a legal and ethical requirement in medical practice to obtain a patient’s informed consent. After the treatment is given to the patient the concept of informed consent is founded on patient autonomy which is a philosophical theory. With regard to the information most of the literature and laws in the area are suggestive of three basic approaches:
- There should be reasonable physician standards in the context of what is implied by such interventions. This permits physicians to ascertain the kind of information that is considered to be appropriate for this purpose. However, researchers have found that most physicians do not consistently obtain the required information in order to focus on patient’s needs.
- There should be reasonable patient standards regarding patient needs in knowing well- informed participants in the decision-making. This aspect focuses upon considering patient needs in understanding the decisions in question.
- Subjective standards refer to what patients should know and understand so that they can make informed decisions. Such standards prove to be very challenging for medical practitioners in incorporating the information for every patient (Delany, 2005).
There is a bond of trust between the medical professional and the patient, and it must be strengthened. It will ensure better treatment by the physician, and the patient too will be taken care of better. Majority of the states in the United States have legal cases and legislation that provide for specific standards in the context of informed consent. The best approach in dealing with these issues is the one that caters to medical professionals’ obligations for giving the best possible care (Browning, 2002). Most healthcare facilities in the US have policies to provide for the kind of health interventions that require informed consent. For instance, procedures such as anaesthesia, surgery and invasive procedures mostly fall under this category. The signed informed consent represents the culmination of dialogues that are required to encourage informed participation of patients in the clinical decision-making process. For a large number of decisions informed consent is neither needed nor required although some meaningful discussions are required to be made. For example, a patient that contemplates about undergoing prostrate treatment, specific treatment pertaining to the prostate cancer is required (Browning, 2002).
Citizens should take part in medical research and be ready to participate in studies that can bring improvement in medical science. In many situations it is considered very appropriate to question the individuals’ ability to take part in the decision making process. In other situations the position is not very clear, and patients are mostly under immense stress as they are ill, and during that time they undergo a lot of depression, fear and anxiety. The high level of stress related with illnesses should not be necessarily excluding people that are taking part in their own health care. At the same time precautions have to be adopted to make sure that patients do have adequate ability to take proper decisions. There are a number of varied standards in the context of decision-making capability. Mostly, the patient’s decision making ability can be assessed by understanding his or her medical situation and the risks associated with the decision in question. Eventually, the decision has to be communicated on the basis of the given understanding. Under situations when this is not clear psychiatric consultation is proved to be very helpful. If a patient does not give consent for a given treatment it does not mean that the patient is incompetent. Patients that are competent to take decisions also have the right to refuse for such treatment even if they are supporting treatments. Such refusals may give directions for pursuing the patient’s understandings and belief about the given decision (Henderson, 2006).
The Health care professionals have to keep in mind the ethical and legal standards while serving patients. These ethical guidelines are the moral standards that govern the health care practices. There are times when the patient’s decision taking ability can vary during different situations. Patients are known to slip from coherent states with fluctuations in medication depending on the intensity of disease. Under such conditions, the medical practitioner is required to get in touch with the patient while he is in a lucid state even if he is required to reduce the medication so that he can be included in the decision-making process. If a patient is not in a position to give informed consent in the context of his health care, surrogate decisions have to be taken in his or her favour. There is a particular hierarchy with efficient decision-makers that are defined by state laws. If proper surrogate decision-makers are not available, medical practitioners are required to act in the best interests of the patients till the time that surrogates are found and designated (Browning, 2002).
The ethical guidelines have promoted the rights of patients to make decisions related to their health. That is why the question for respect for autonomy has arisen. Issues relating to informed consent continue to be of immense concern for medical practitioners in view of the fact that people have become educated in the context of their legal and personal rights. Large-scale malpractices claims that result from surgical procedures have given way to the need for genuine understandings of the role of nurses in pre-operative matters. Nurses have to be provided to each patient in the context of their liability and matters relating to the informed consent because they provide high levels of health care and perform a large number of invasive procedures. Matters such as patient competency in understanding and signing surgical consent should be of immense concern for nurses (Sypher et al., 2005).
The physicians must follow the ethical guidelines strictly as it tells upon on the importance of informed consent and respect for patient’s autonomy. There is a constant struggle for economic resources that has mandated the future plans and policies in healthcare that are continuing in considering patient rights while providing care. In this context, consent is understood as voluntary agreements by people that have appropriate mental capability in making intelligent choices about the actions that are proposed on them by another person. Such actions relate to the administration of medicines or performing specific medical procedures on patients. Issues relating to informed consent can significantly alter actions of touching in the context of nonconsensual to consensual actions. However, it is recognized that informed consent is not required for routine procedures such as Angling touching and bumping that can occur during surgical procedures. Informed consent is also understood as the medical practitioner’s legal duty to provide all the information to his or her patient so that the patient is allowed to understand the process before giving consent for surgery. Informed consent also requires that there should be complete recognition of the risks, processes, and the probable outcome in patients that are undergoing treatment (Sypher et al., 2005).
In informed consent it is the duty of the physician to present different alternatives to the patient. The physician must give the patient the information about the pros and cons of the options that are available and then proceed with the treatment that is selected by the patient. There are several general issues related to informed consent and it is evident from court decisions that the responsibility of informing and warning patients lies with physicians and nurses in ensuring that they have been fully informed of alternative treatment options and complications pertaining to the present treatment prior to signing of the consent forms. The relevant law requires that medical professionals should inform patients about the medical processes that could impact on normal people. This is also known as prudent patient standard and many states follow the prudent physician standards whereby complete disclosure is ascertained by way of what medical practitioners would do in any given situation. Moreover, when full disclosure is determined it implies that certain facts have to be presented to patients, like nature of illness or injury, the process to be performed, the object of the process and the probable risks and consequences of the process. The patient also has a right to prove the probability of success, other possible treatments and the risks associated with not receiving the given treatment. There has to be an indication of the patient’s recognition of such issues and the document should have signature of the patient, the doctor has to verify the fitness along with the date on which the signatures have been put. It is important to ensure that healthcare providers give importance to such principles of informed consent so that malpractice claims and liability is avoided (Maxwell J. Mehlman, 2011).
There is always an alternative in the informed consent. Even if there is only one option available to the patient, he or she can still choose to do nothing. Informed consent maybe simple in theory but it does raise a number of questions. What are the alternatives that a physician should tell the patient about? If informed consent is not obtained properly from the patients it could invite litigations relating to negligent acts. In such cases the Judiciary determines if any breach of conditions has been caused by the injuries. But the court has to define how there is a reasonable relationship amongst the extent of injury and breach of duty. During times when patients feel and autonomy of choice becomes uncertain due to the lack of understanding, a number of legal conditions may apply, such as consent permission, complete events, voluntariness, comprehension and disclosure (Maxwell J. Mehlman, 2011).
According to the legal standard that is followed, the duty of a physician is to inform the patient whatever is expected of a reasonable physician. If there is a dispute over whether the physician obtained the patient’s informed consent, then in that case both parties need to bring expert testimony from other physicians and state the things that a reasonable physician must tell a patient in such a situation. Theorists hold that the duty for disclosing information about surgical procedures and for obtaining informed consent lies with physicians. For instance, there are several cases that involve patients that were given neurolytic blocks by making use of phenol such as epidural injection were provided for in the consent form of the patient. It is also observed in such cases that patients do not mostly get consent form. In such cases courts have ruled that even if patients are provided with legal disclosures, they must understand what the physicians want to say and figure out the information given in the consent form so that permission can be given voluntarily for surgery or treatment (Maxwell J. Mehlman, 2011).
Concept of informed consent
In order to legitimize the imposition of the recent technological risks informed consent is a concept that has been proposed in the field of medicine. This concept was introduced to assure and safeguard the autonomy of the patient. In the context of informed consent, voluntariness refers to freedom of choice without using stress or coercion and although the concept is difficult to define, it is crucial in issues relating to informed consent. It is known that human interaction involves complying with certain pressures but medical practitioners cannot give any explanation by way of extreme terms and enforce on patients to make any choice that he or she would not be inclined to make (Asveld, 2006).
There is no one-solution for physicians and patients to know that how much information is enough and what is the best way to describe the potential risks to patients. But there are certain exceptions in emergency situations whereby patients may have to be treated against their will. Such treatment is given when in action on the part of medical practitioners would lead to more harm to patients and the patient may be unconscious but requiring emergency treatment. When the patient is conscious and does not give consent for getting life-saving treatment, medical practitioners can hold such treatment till the time the patient becomes unconscious. But it is essential for medical practitioners to prepare adequate documentation while carrying out medical treatment without the consent of patients (Lustig, 1996).
Informed consent can never be a process or an event that can be just reduced to “one size fits all”. Competency has been defined rather vaguely by courts but certain levels of standards to prevail in the context of determining informed consent and signing on them. The legal practitioners and nurses have to attain more knowledge in the context of laws in different states and also in the area of competency. Aged patients would be suffering from hearing impairment, slow patterns of thinking and the presence of transient confusion. Many aged patients suffering from stroke often suffer from difficulties in speaking and thus require additional time for effective communication. Many patients often refuse to give answers to questions because they are not satisfied about being in hospital. Though any of such issues can be involved, if patients are encouraged to paraphrase what they understand about the given processes it proves to be the most effective way in determining whether they are aware of the provided information. Thus, patients are able to understand the benefits and associated risks. However, such factors do not necessarily imply that there is no competence or decision making capability amongst health care professionals. Under situations when the capacities of patients are doubted by medical practitioners, they usually derive informed consent from the nearest relatives. It is obvious that assigned informed consent by the patients is a better option as compared to not having any consent at al (Lustig, 1996).
The Legal Model of Informed Consent
The disclosure model of informed consent has many shortcomings and ignores the linguistic complexity of any communication act. The physicians are dealing with increased risk of difficulties in some special circumstances related to informed consent. Before any insidious tests can be performed all medical treatment that are provided by the physicians need permission from patients in ways that are completed, voluntary and well-informed and this process is known as informed consent. Every patient has the right to seek appropriate information about the benefits, risks and alternative treatment modes while making decisions about medical treatment or care. If the patient is not in an appropriate state to understand such concept, the physician has to seek permission from a person who is named by the patient as holding a concrete power of attorney in the context of healthcare. If nobody is available, then the physician can seek the help of other authorized decision-makers (Marta, 2011).
According to Joubert et al. (2005), self-determination regarding informed consent implies that people of sound mind are at liberty to take decision about what is to be done to their body in terms of healthcare. Ethical and legal doctrines are founded on the strength of informed consent. Essentially, the procedure pertaining to informed consent has to stem out of discussions amongst patients and physicians. Patients are required to seek information about their medical condition and the options available for treatment, while doctors are required to provide information and insight along with the relevant advices and support that are available to such patients, and doctors who have the duty to provide information are easily understood by the patient, and the associated benefits and risks are also communicated effectively. When it is required by law, the medical practitioners must take appropriate steps to provide the required information and remove communication barriers.
Informed consent is considered to be significantly achieved when people are able to understand their current medical status more and the treatment that is to be followed. Patients should be able to benefit from the available treatment after being explained about the possible risks and advantages of the treatment. Mostly, patients are provided with the best possible alternatives and informed about the uncertainty associated with all components of the given treatment. Usually a summarized document contained a brief of the discussion which is signed by patients for all major decisions pertaining to ethical treatment (Katz, 2003).
The right to informed consent is also associated with the right of informed refusal, because patients who have clinical and legal capacity will have the right to refuse any kind of medical care. They can refuse such treatment even if it is usually acceptable by others and is proved to be specifically life-saving. For instance, a person suffering from a strong heart attack is open to take decisions about leaving the hospital even if such action is likely to create severe risk of death for him. It is because the person decides to abstain from medical treatment does not mean that it is evidence of the person being incapacitated. There are several instances whereby people have refused treatment on the basis of fear, lack of trust and misunderstanding. However, refusal could also be the result of other medical and psychological conditions such as delirium and depression. When a patient refuses to have medical care that medical practitioner is prompted to make further attempts by discussing with the patient. Any patient’s well thought refusal of getting treated is not considered to be attempt to suicide. At the same time, when a doctor complies with the persons who have not given consent it cannot be legally considered as assistance towards suicide. Instead, the death that follows is legally understood as an actual impact of the disease or ailment (Juengst, 2004).
Katz (2009) has held that there are times when a patient’s refusal to get treated can cause harm to others. For instance, patients who refuse to get treated for certain infectious diseases create risks for other patients in the context of risks of infection. Similarly, with patients who refuse to allow treatment for other people namely, a dependent adult or a minor child, are considered as placing that person’s health at risk. Under such circumstances doctors are expected to consult experts, judges and lawyers of ethical practices. From the legal perspective, informed consent simply implies a patient’s signature on a consent form. It primarily relates to the process of communication amongst medical practitioners and patients that eventually leads to the patient’s consent and agreement to go for a specific kind of medical intervention. In such communication processes, physicians who provide and perform the medical treatments and procedures are required to provide more appropriate information and have to discuss with the patient in the context of:
- The known diagnosis of the patient
- the objective and nature of the proposed procedure and treatment
- the benefits and risks associated with the proposed line of treatment
- alternative modes of treatment available to the patient irrespective of the cost or the manner in which the treatment are covered under health insurance
- the benefits and risks associated with alternative forms of treatment
- The benefits and risks associated if the patient opts not to deceive or undergo the given treatment.
Such practices allow the patients to get the opportunity to seek information in order to have a thorough understanding of the treatment process whereby he or she is able to take concrete decisions for proceeding or refusing the given modes of medical interventions. Such communication processes or deviation from them are both considered as ethical obligations and legal requirement that are provided for in the case laws and statutes of all the states in the United States of America. Although the legal concept of informed consent is quite recent, the medical practitioners have an ethical obligation to provide patients with relevant information and guidance regarding the given medical treatment (Wang, 2000).
If medical practitioners wish to protect themselves from avoidable litigation it is meaningful for them to document all the communication processes with patients. Efficient documentation serves as effective evidence in confirming that the due process of law was followed while discussing with the patient (Kuper, 2006). If timely and complete documentation is done by physicians providing the given treatment or performing the relevant surgical process, strong evidence is created for support of such medical practitioners. A consent form that is well-designed proves to be useful for medical practitioners, but if it is excessively exhaustive and detailed, it may work against the interest of the medical practitioner. Consent forms are required to mainly serve all legal formalities by providing information to the effect that all material risks are explained and discussed with the patient. But this requirement can prevent patients from stressing that actual disclosures did not include the explanation of associated risk that was discovered by the patient after he received treatment. Conversely, it is also marked Weiss for practitioners to list all the risks in the consent form because an exhaustive listing can prove to be complicated for the patient to understand why things that are omitted from the list will probably be considered as being undisclosed. If doctors make use of forms that contain the list that is to be explained to the patient, they must include the language that is spoken by the subject. Medical practitioners providing health care are required to be aware of consent requirement which is supposed to be general knowledge that can be used when they seek further information from professional consultants and attorneys (Kuper, 2006).
Social researchers have been able to make correct interpretation of ethical guidelines to meet the requirements of their research projects within the regulation and legal frameworks that impact the manner in which informed consent and research ethics are handled. This happens specifically in the context of research relating to children in health care settings. In regard to legal frameworks informed consent is considerably impacted by the Data Protection Act 1998 and Article 8 of The Human Rights Act 1998. It is also known that the human rights Act is protective of rights relating to respecting private and family lives and is therefore supportive of the need for informed consent in research activities. In the context of the Data Protection Act, some aspects that relate to informed consent information and disclosure of data to researchers of potential participants should not overturn the rights relating to individual’s consent in the given circumstances. This also assumes significance in view of the manner in which the data is limited for which consent is usually sought for storing the relevant data. In the context of research relating to children the prevailing law is quite complicated and is related to the concept of competence (Walton, 2010).
In many states of the US, children under the age of 16 are not provided by law to be legally competed for giving informed consent. But the child can be assumed to understand the kind of participation in research activities and under such circumstances parental consent is not at all considered necessary. The concept is based on assumptions that children who have appropriate understanding can give informed consent, and therefore under such conditions parents do not have rights to overrule their children’s desires. It is noteworthy in this regard that making assessment of the competing level of children is not a straightforward exercise because the understanding and attitude in the matter of competing is quite diverse amongst researchers. Moreover, assessment of competence levels is specifically dependent upon the complication and risk associated with the research that is being carried out (Walton, 2010).
Researchers have observed that one method of resolving such issues is to make assumptions that school going children have competency to give informed consent and the responsibility then lies on parents if they disagree to prove incompetence of their wards. They have also argued that researchers should not be subjected to risks relating to legal action introduced by parents of children that are below 16 years old. It is also true that researchers are at risk if claims of harm are made by children. Some researchers have observed that in the context of controversial areas of research, very few amongst them are likely to be contested in cases where researchers have exercised prudence in seeking parental consent before conducting research. There have been no instances of such cases having been initiated against researchers in social science. In this regard, The Children’s Act of 1989 is considered to be quite relevant in the context of informed consent in relation to research activities carried out on children (Walton, 2010).
However, this is primarily related to matters relating to confidentiality in cases where children are considered as being in danger. In such cases also the researchers are not held because children over the age of 16 years are considered to be competing for giving informed consent for their own treatments. But, informed consent from guardians, parents and other relatives is usually considered to be necessary in matters relating to research activities on children and adults that are not competing to give consent for their own treatment. Such categories of people including those that suffer from learning disabilities and mental health problems who are unable to fully understand the implications of taking part in given research projects would be devoid of the impact and consequences of such involvement in research. It is observed that many courts have considered that people having paid into responsibility can give informed consent for research activities of other individuals as far as it is not against the interests of the individuals and does not impose unwarranted burden on them. However, the legality of research studies in the context of incapacitated adults and children has not been contested in most courts, and the department of health has held that research activities involving such people should not be considered the same as in the case of research carried out on people that are competing to give informed consent. There are a number of variations in the manner in which social research activities have been conducted and managed while considering these issues within the legal context (Walton, 2010).
The legal regulation of consent in USA, UK, Europe & Middle East
The United States has introduced informed consent laws to regulate certain communication amongst medical practitioners and patients. These laws provide for some information that patients have to be provided with, in order to enable to make informed decisions about undergoing specific surgical or medical treatments including diagnostic studies. These laws are equally applicable to physicians, surgeons and the nursing staff. The law, however, varies from state to state in the US. Some states provide for stringent regulations that govern specific situations. For example, certain states provide for specific information about the matters relating to the treatment of breast cancer and for clinical trials relating to scientific research of newly introduced means of treatments. Another variance relates to the fact that they require only reasonable information, while others provide for compliance in providing full and complete disclosures. In this context, disclosure means making the relevant information known to patients. Generally, informed consent issues assume that patients are legally able to make their own decisions about informed consent for the given treatment. If a patient is not legally competent to give informed consent he or she has to be represented by a family member who is legally permitted to make such decisions. He or she has to go through the same process on the patient’s behalf (Kaufert et al, 2005).
If informed consent is to become legally valid, the provided information has to be well understood by the patient in question. Such responsibilities are shared by the patient, because the medical practitioner will not know what the patient does not understand until he is asked about the specific information of the treatment or procedure. As per provisions of law the patient has to be given the opportunity to consider the information and to ask any questions that come to his mind. Informed consent assumes that while making decisions, patients are not supposed to be coerced in any way so that they choose on their own about the treatment that they feel is best suited for them (Kaufert et al, 2006).
Federal Regulations and Guidelines Pertaining to Informed Consent
The DHHS-conducted/supported research on human subjects is primarily regulated by 45 CFR Part 46. The DHHS regulations describe both when informed consent is required and what elements must be put in an informed consent document. As custodians of human specimens, bio-specimen resources should track whether appropriate informed consent is present (if applicable) or the reason why informed consent is not necessary (Baker, 2006). The OHRP has issued guidance on regulatory requirements that must be satisfied by bio specimen resources. The OHRP recommends that the following be included in informed consent documents for bio specimen collection:
- A clear description of the operation of the bio-specimen resource. This description could include details that may be of interest to human subjects, such as whether identifiable information will be maintained in the bio-specimen resource and/or whether research results will be linked to the bio-specimen.
- The conditions under which data and samples will be released to recipient-investigators. Procedures for protecting the privacy of human subjects and confidentiality of data.
- Where human genetic research is anticipated, information about the consequences of DNA typing (Benedetto, 2005, p. 872).
The informed consent document should describe how human subject data will be used and stored. Where applicable, the informed consent document should state whether identifiable or coded information will be maintained in the bio-specimen resource and if research results will be linked to other data about the human subject, such as clinical data obtained from anatomic pathology and clinical pathology laboratory information systems and cancer registries. If longitudinal data is collected, the informed consent document should state that the subject’s medical records are accessed for this purpose. Informed consent documents should describe whether the bio specimens and/or the data associated with or derived from bio specimens will be shared with other investigators and, if so, the oversight mechanisms for such sharing (Baker, 2006).
It has been argued by researchers such as Kaufert (2004) that if individuals take part in research activities when it eventually proves to be in the interest of society, instead of believing that opting to become subjects in medical research is a sacrifice, participation in such activities should be viewed as being beneficial to society. It is mostly assumed that medical experimentation is risky as compared to traditional treatments. Many researchers argue that high standards of informed consent are necessary for research as compared to medical treatment. Most of the medical treatment is considered to be as risky as research is carried out on patients. Medical professionals who conduct experiments are obliged to comply with straight processes and have to be ready to take care of any complexities of side-effects that may arise due to such experiments (Kaufert, 2004).
Prior to conducting human trials, medical professionals are required to have strong reasons that the given experiments can be of immense help with very less chances of harm being inflicted on subjects. On the other hand, many traditional treatment systems have not been entirely proved to be of much benefit and medical professionals have been found to avoid such problems. Hence subjects in medical research are not required to be altruistic but are encouraged to volunteer considering their sense of responsibility towards society and on behalf of their self-interest. There is a strong need to focus on informed consent and related to qualitative research that comprises of conventional methods of data collection, such as observations and interviews. Such efforts are not required to involve with the large number of complicated ethical issues in relation to the research that uses modern digital technology and visual processes. It also does not involve the matters relating to consent related to quantitative research. It is thus important to explore problems relating to informed consent in qualitative social and medical research, particularly to focus on research that is carried out on vulnerable groups that include children, aged people and people that suffer from physical and mental health complexities (Kaufert, 2004; American Psychiatric Association, 2007).
Table 1. Readability scores of informed consent forms in research approved by the
Massachusetts Department of Mental Health
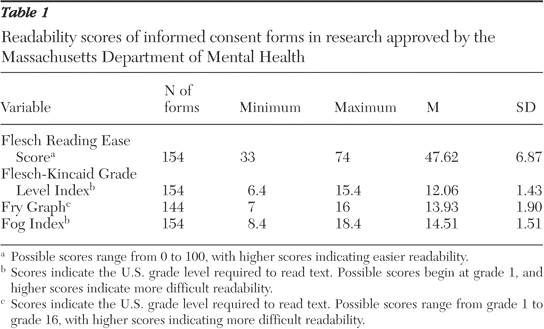
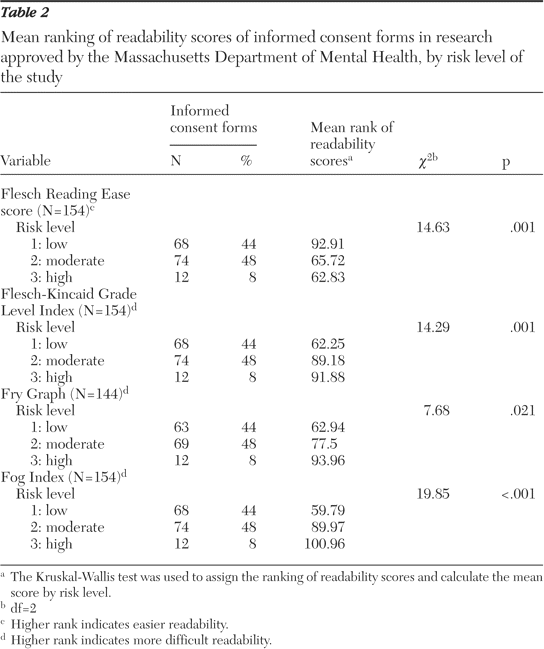
In many situations it is considered appropriate to question individual’s ability to take part in the decision making process. In some situations the position is not very clear and patients are mostly under immense stress because they are ill and are undergoing a lot of depression, fear and anxiety. The high level of stress that is related with illness should not be necessarily excluding people who are taking part in their own health care. At the same time precautions have to be taken to make sure that patients do have adequate ability to take proper decisions. There are a number of varied standards in decision-making capability. Mostly, the patient’s decision taking ability can be assessed by understanding his or her medical situation and the risks associated with the decision in question. Eventually, the decision has to be communicated on the basis of the given understanding. Under situations when this is not clear, psychiatric consultation is proved to be very helpful. If a patient does not give consent for a given treatment it does not mean that the patient is incompetent. The right to informed consent is also associated with the right of informed refusal because patients that have clinical and legal capacity have the right to refuse any kind of medical care. They can refuse such treatment even if it is usually acceptable by others and is proved to be specifically life-saving. In clinical trials informed consent is mostly required to be inclusive of all details and thorough as compared to a consent that is given for standard medical procedures and treatments. Such rules have been introduced because there are more possibilities of uncertain impacts with new treatments. In the UK, The Medicines for Human Use (Clinical Trials) Amendment Regulations were introduced in 2006 to include conditions where it is not possible to get informed consent for participants that take part in Medicinal Clinical Trials. However the legislation has taken care of only clinical trials and in other aspects Informed Consent has to be obtained from patients taking part in research activities. In some cases where informed consent is not available or possible to get, approval under Section 60 has to be taken by medical practitioners (Lock, 2007).
Other regulations in the UK have described provisions in the context of adults and minors that are not able to give consent for them and also include the roles and duties of legal representatives. As per directives of the EU, there are some basic principles in the context of incapable adults and minor patients relating to medicinal and clinical research activities. Collste (2007) states that:
Persons who are incapable of giving legal consent to clinical trials should be given special protection. It is incumbent on the Member States to lay down rules to this
effect. Such persons may not be included in medicinal clinical trials if the same result can be obtained using persons capable of giving consent. Normally these persons should be included in medicinal clinical trials only when there are grounds for expecting that the administering of the medicinal product would be of a direct benefit to the patient, thereby outweighing the risks (Collste, 2007, p. 236).
When people are not able to give informed consent such as patients suffering from dementia and those suffering from psychiatric conditions, it is required that they should be included in clinical trials on a very restrictive basis. Drugs for treatment in clinical trials have to be given only to such individuals who are likely to directly benefit by outweighing the associated risks. Additionally, in such circumstances the informed consent from the individual’s legal representative is to be obtained strictly in consultation with the attending physician before the individual can take part in the clinical trials. The concept of legal representative relates to the current national laws, and therefore it can also be inclusive of legal or natural persons comprising of authorities or bodies duly provided for in the country’s laws. The UK Medicines for Human Use Regulations 2004 gives legal definition of legal representative in the context of clinical trials. However the definition varies if the subject happens to be a minor or an individual that is suffering from any incapacity. The definitions of legal representative also differ in circumstances when the individual is suffering from incapacity and located in Scotland. The law in the UK specifically provides that the concerned person should not be an individual connected with the conduct of the trial (Leach, 2005).
Regulation and ethical review
In the United States, researchers are required to comply with regulations or, the National health scheme, and at the Research ethics committees that has had considerable influence, on the methods adopted by researchers in the context of informed consent. All research activities, relating to patients and staff, conducted by the medical practitioners must have approval from the ethics committee of the National health scheme. Moreover, a research governance frame work has been developed that runs parallel with the National health scheme research committees. The changes have led to a strong bureaucratic system whereby ethical approvals of procedures have proved to be quite burdensome and time-consuming for medical researchers as well as social researchers. As a consequence of such criticisms advisory committees were formed to streamline the systems.
Social research in the context of informed consent has been largely subjected to ethical reviews by institutional research ethics committees that have considerably increased in number in the last few years. Recent surveys have indicated that many universities are in the know of the need for this kind of committees and had started introducing procedures for ensuring the ethical investigation of research that involves human subjects. It is also possible that the given regulations will be enhanced in the future and although such prospects have been received with considerable opposition by social scientists, many have highlighted the advantages associated with regulation and pressed for social researchers to get engaged in the process of developing better systems for informed consent. Therefore it is not surprising that ethical reviews will soon become a necessary requirement for funding institutions (Lock and Gordon, 2007).
Ethical regulations of social research is considered to be a state subject with changes happening as a result of large number of institutional, moral, political and legal impacts that have been evolved very rapidly. Researchers have now become aware that they had to keep updating themselves in the context of the need for more ethical reviews in their institutions as also in the context of the requirements that have to be met towards funding organizations. Angell (2006) states that:
However, there is a need for medicinal clinical trials involving children to improve the treatment available to them. Children represent a vulnerable population with
developmental physiological and psychological differences from adults, which make age and development related research important for their benefit. Medicinal products, including vaccines, for children, need to be tested scientifically before widespread use. This can only be achieved by ensuring that medicinal products which are likely to be of significant value for children are fully studied. The medicinal clinical trials for this purpose should be carried out under conditions affording the best possible protection for the subjects. Criteria for the protection of children in medicinal clinical trials therefore need to be laid down (Angell, 2006, p.968).
Although informed consent appears to be quite straightforward at first glance and relates to more appropriate knowledge and information so that people can make an informed decision about participating in research projects, a detailed analysis of the related issues has revealed that the procedures are not entirely straightforward. Researchers have argued that the true concept of informed consent applies to cases where subjects are provided with full explanations and have the ability to achieve a thorough understanding of their participation. However, it has been found that such situations will relate more to rhetoric than to realistic situations. Some researchers have provided practical explanations as to why such situations could be true, especially in the hardships of making subjects to understand (Macklin, 2003).
Many subjects have very remote chances of apprehending the adversities of participating in such research efforts while giving informed consent. However, such researchers have also argued that in view of the tension that prevails amongst the subject’s right to give refusal and the researcher’s motivation to succeed with the subjects, different strategies are used by researchers to motivate participation amongst individuals. In view of such views by different researchers, the work on informed consent has raised a number of significant questions in the current text of research on different issues while reflecting upon their own perceptions of informed consent procedures. Social researchers are required to have a balanced approach in the matter of the different factors while dealing with the issues relating to informed consent. They are required to adhere to several legal regulation and frameworks and are also required to balance several issues such as the objective of the research, and what they believe to be in the best interest of individuals participating in the research. They have to keep in mind the interest of formal and informal gatekeepers in addition to being reflexive about different matters relating to competence, consent and information (Macklin, 2003).
The provision relates to giving information through which researchers have two negotiate delicate balance while giving information to subjects. They are required to give exhaustive information so that participants are able to make concrete decision regarding their participation. It can be argued that providing information should include details about the research study and the researcher’s viewpoints and the source of funding for the research program. However, some researchers have noted that there is need to avoid the provision of information by researchers in a manner that the same does not put off participants. There is need to take decision about the style and manner in which information can be provided before it can have a negative impact upon people who have agreed to participate in the research. Researches in psychology are throwing light on different ways of providing information that impacts on the understanding of people, which in turn could influence their willingness for participation in the research program (Macklin, 2003).
The provision of information usually includes written information in collaboration with or in sequence with the provision of oral information. It is important not to overwhelm research subjects with excessive information. They should be provided with information that is attractive and friendly and meaningful. This objective requires researchers to pay attention to give specific importance to the layout and the kind of language that is used in the graphics. There is a strong need to avoid information that appears to be official although different kinds of ethical regulations have mostly constrained the ability of researchers to achieve such objectives, specifically in healthcare settings. Information sheets that appear to be official documents and that specifically focused upon confidentiality have the potential to create distress amongst participants and are considered to make individuals reluctant from participating in medical research. Such circumstances have negative impact on dependent groups by encouraging family members of patients as well as health-care workers to refuse participating on their behalf (Macklin, 2003).
There are several research studies that wish to make use of socially excluded or hard to reach groups, but the style and methods of providing information is more important and the significance of working together with the community is considered to be crucial by researchers. This is a major issue in research studies that address vulnerable groups. For instance, researchers and childhood researchers that work with people having a learning disability have revealed the significance of maintaining minimum written information instead of including graphics and pictures in the information provided by them. Researchers have also made experiments in different ways by giving information for meeting the requirements by providing the use of computers, videos and photographs. In many fields and kinds of research it is possible to provide only oral information. Such circumstances occur in situations where the formality of providing written information is not considered appropriate for specific groups such as those relating to illegal activities. They could also be the element in settings that are not conducive to the reading and written information as provided to potential participants. Many researchers have observed that they have to be very cautious in the context of minimizing the extent of information given by them (Macklin, 2003).
Many participants in research activities have been keenly taking part because of their immense interest in the given topic and because they do not wish to be viewed as uncooperative. They also do so because they may not be aware of the involved risks direct participation in such research activities. In such studies most of the participants do not give much attention to the explanations offered by researchers in the context of the involvement of the research. Moreover, they may not be agreeable to find time or to take time for reading the information sheets. Therefore it is very important for researchers to understand the information requirement of the groups that wish to research. It is also important for them that appropriate knowledge be used for providing information in ways that allows potential study subjects to recognize how participation will impact on them (Macklin, 2003).
Many researchers who are closely involved in the field of childhood research and have also been associated with other vulnerable groups have observed that this kind of research does not necessarily impose any significant tactical or methodological obstacle. Many social researchers consider that it is quite possible as well as preferable to provide information infeasible banners that allow future study subjects to recognize the impact of participation and involvement in different levels of capacity in research activities. Such researchers have observed that they feel it obligatory to find ways of allowing people of different age groups and competence levels to give informed consent for participating in research activities after they had given appropriate information. They have concluded this after confirming that such information can be easily understood by subjects in the given research settings. For many researchers this involves creating collaborations with future participants in order to ensure that their viewpoints are given due respect (Mappes and De Grazia, 2005).
In order to achieve such objectives many researchers have started working closely with peer research groups involving children and service users show that they are ensured and are being provided with information in the desired ways. The issue of withholding information assumes greater importance because technocratic and observational research in patient could be intentionally or unintentionally withheld during the research. Some observational studies have revealed that it is quite difficult for all participants to be informed adequately about all issues. For example, if the observation is being carried out in public places it becomes practically impossible to give information to all people that are present in the area. In the context of other research studies such as in homes or residential part it is possible for researchers to keep patients, staff and residents informed about the observation and research study (Mappes and De Grazia, 2005).
Moreover, it is possible to inform people by way of display strategies although other people can enter the research study that have not been made aware of the same till that time. Many researchers argue that it is not proper to seek and provide information for participation in research studies if people want come to know that they are subjects of research studies, because of which there is all likelihood that they may change their behaviors. Several studies in psychology have been following such models though researchers have been providing information about participating in this study only instead of focusing on providing complete information. Many researchers have been taking a stronger stand in arguing that if information is withheld it is appropriate to take consent from participants because that appears to be the only means by which organizations, institutions and several areas of society can be made open to all elements, which is also in the interest of the public. Researchers that favor covert methodology in this regard argue that this approach does not necessarily bring harm to participate and that the so-called open research methods mostly make use of practices that relied on deceitful methods of obtaining information. At the same time there is extensive criticism of covert researcher because they are believed to have the same objective and can be pursued by using open methods (Mappes and De Grazia, 2005; Marshall et al., 2006).
High levels of research have strongly restricted the ability of researchers to carry out covert research and to provide only verbal information without obtaining signed consent from patients (Marshall et al., 2006). This aspect has been of immense concern amongst researchers when the information has to be provided. The difficulty arises because providing information pertains to the fact that in qualitative research there is strong focus on data collection that considerably impacts upon the outcomes of the research study. As it is not known at the time of commencement of the research, it is implied that the focus of the research will be made on the number of participants and the pattern of interviews that will be conducted with every participant. In the context of receiving specific direction the research will be inclined to take a specific direction in being dependent upon the collected data and the analysis that will emerge from the given information. Such patterns are developed in areas of technocratic research. And in order to give information and to obtain consent from participants at the start of any research study. These issues have been outlined by several researchers in the context of their ethnographic research in hospital settings. They found that because of changes in the conditions of patients they are not readily able to say whether they are still willing to participate in the research. Some patients had difficulty in remembering the researchers who had to strike a balance in providing information (Marshall et al., 2006).
In view of such circumstances researchers have often used practices overt participant observation because it is known to be less intrusive in terms of collecting relevant data. It has been pointed out that the line that is drawn in by bifurcating covert and covert research can often become considerably blurred. It has been argued that information should be provided by researchers and they should seek consent every time they start collecting data from research participants in order to make sure that they are in the know of information that has been collected from them and that they are ready to continue with their participation in the research. At the same time the procedures by which such objectives or achieved can become quite difficult because researchers have observed that participants may not be willing for long to continue with their participation in the research. This also implies the significance of researchers to balance and provide proper information in meaningful ways to ensure that the provided information does not discourage people from participating. Many research organizations and researchers adopt practices of freight material or financial rewards to participate in research studies. However, the information to provide to participants in such studies relates mainly to personal information of participants. Although such patterns are seen as inducement that can be considered as a form of caution that influences the voluntary aspect of research participation as there is limited consensus in the context of appropriateness of such payments and rewards that are given to participants in research studies. Some researchers are of the belief that all participants should be given payment for the time that they provide and efforts that are made (Marshall et al., 2006).
Researchers such as Moreno et al. (2004) are of the belief that the practice of giving such rewards would make potentially vulnerable people to take part in research activities for the wrong reasons. Such situations become specifically complex when participants comprised of people that come from lower class backgrounds or when they comprise of people that are not financially sound at a given time. Many researchers manage such situations by not providing complete information to people that they planned to pay for their participation in the research. Instead, it refers to give payment as a gesture of thanks after the research has been completed. Obviously such practices is not possible to keep this element of surprise for a long time as the word spreads around amongst participants very soon, especially when they are invited from limited communities. The practice of giving rewards and incentives does not necessarily comprised of gifts or money because many research projects have been providing a variety of incentives, compensation and rewards for the effort and time provided by participants. Providing information into such studies is closely associated with the issue of obtaining informed consent of participants. There are several issues that have to be addressed by researchers to ensure that people are given the opportunity in considering if they wish to participate in the given study. The ethics committee often expects that a minimum of 24 hours should be given as time for them to consider if they wish to take part in research studies (Marshall et al., 2006).
There is considerable variation in the context of viewpoints helped by researchers about the significance of signatures of patients as evidence of informed consent. There is considerable expectation about the fact that research studies are at an advantage if they obtain signed consent from participants in medical research studies, which is mostly viewed as something important. The advantage of making use of signed consent form increases the chances that the participant will understand the risk in participating and what is involved in terms of his or her rights and other issues such as anonymity and confidentiality. Moreover, the signed consent form is considered to be protective for the research in anticipation of possibilities of allegations by study participants. Although the participants and nature can be considered as important for safeguarding researchers as real as the participant, situations can become quite problematic in some aspects, especially, during the research that is related to the main behaviors and socially unacceptable research studies (Nuffield Council on Bioethics, 2002).
Hurdles are also created in situations where research participants need to be protected. Moreover, the requirement of obtaining signature in other perspectives can also originate problems because it implies that the process becomes former, which is eventually feared in putting off some participants. There are additional problems in the context of how signed informed consent forms are to be dealt with in the context of participants that are not literate or those having communication and language difficulties. Such issues are very important for researchers who worked with participants in suffering from learning disabilities and consequently researchers have found different ways of obtaining informed consent by forging or avoiding the use of their signatures. Such measures include seeking recorded consent or by marking the consent form appropriately or by using different colored cards to seek response of yes or no from participants. Researchers have also observed that using signed informed consent forms could lead to the compromising of different issues relating to anonymity and confidentiality, which are considered to be significant issues when research participants need to be protected. At the same time participants could also develop fears that signing informed consent forms could allow the provided information to be traced to them, thereby putting them to avoidable risks of physical harm. Such issues relate to research areas dealing with domestic violence. Participants are also fearful that they can become vulnerable to possible prosecution and investigation by law enforcement agencies, especially in the context of illegal activities. Researchers have noted that people may wish to rotate their identity from researchers and expect them to pass on the information, which is contrary to ethical principles. They hold that people involved in illegal activities that are required to sign informed consent forms, are unlikely to give their consent for participating in research activities and even if they do give their consent they will give incorrect names thus making the process without much meaning. Further, signing consent forms are not considered to be in the interest of researchers who could force them to take part in prosecuting such participants, thus undermining, and contravening the responsibility of researchers towards their participants (Nuffield Council on Bioethics, 2002).
Many researchers use proxy consent while considering vulnerable groups that are lacking the capability of understanding the significance of research participation. They also believe that such participants are not mentally fit for providing informed consent for their own treatment. Most research studies that have used proxy consent have involved with mental health services users, children, people with learning disability, infirm and old people. Most participants that provide proxy consent are mostly care givers and relatives of the patient. Informed consent by proxies has the potential to be used for examining the record of the individual for the purpose of observation and for conducting interviews. Many researchers feel that proxy consent is not appropriate and should be avoided as far as possible. It is quite obvious that ethical review committees focus upon wanting strong justification for using proxy consent. If this does not become possible, inclusion of individuals who in research studies create considerable ethical issues that require strong justification. In this connection it should be noted that informed consent does not necessarily mean that it is limited to giving consent for collecting data during the given time and place. Researchers that have worked in participatory paradigms believe that it is not proper to provide transcripts to research participants in order to allow them to confirm that they are satisfied about what they have informed during the interview, which can be further included in the research study. Researchers who have worked on such patterns are quite satisfied in making amendments to transcripts provided to participants. However, many researchers have also objected to this practice and considered that transcripts that are generated from research belong to the researchers. Moreover, after the collection process of the data has been completed, they believe that the subject should not be given any authority to question as to how such data was used. Researchers believe that informed consent should be extended to more content (Nuffield Council on Bioethics, 2002).
It is normally assumed that participants wish to be treated as anonymous. This practice has been frequently questioned by several researchers. A research that was conduced amongst children in palliative care have held that research participants mostly wish to have their own names to be used and that it must be clarified during the time when consent is obtained from participants. Such issues have also cropped up while using visual data such as photos and videos. Another issue that has been raised relates to consent for using the information as archives. Qualitative data proves to be an important source for reusing and archiving important information, but signed consent forms from study participants have to be collected for such purposes. Informed consent should be generally obtained after the process of data collection is completed so that individuals know about what the data will comprise of in the context of their anonymity. It is mostly expected that the consent form and information sheet should provide information that participants have the right to withdraw from at any given time. In this context researchers have observed that it is quite usual, particularly in the case of specific groups, to restrain themselves from stating that they do not wish to continue being included in any given project. For instance, it might prove to be difficult for children to tell adults that they are no longer required to participate in any given study or that they do not wish to give answers to specific questions. The same issues also apply to people in different context in view of the power relationship that exists amongst researchers and participants. At the same time, they can be considerable lack of awareness of the freedom to say no to anything that they may have agreed to previously. It is observed by researchers that there is strong need to be careful about the unspoken expressions of participants’ unwillingness to continued participating in the data collection exercise. There have been instances where there is apparent lack of interest during the data collection process. During research processes involving children and people with restrained communication, researchers have successfully used cards of different colors that can be used by participants to convey the decision if they do not wish to give answers to specific questions or if they do not want to participate in the research anymore (Nuffield Council on Bioethics, 2002).
The role of gatekeepers in this regard is quite significant in that the text of consenting vulnerable people. The issue of the capability of people such as young people, children and patients with mental health problems and learning disability has assumed considerable significance because they are unable to give informed consent effectively. It has been observed that in research studies where researchers have been given the freedom to give information to possibly vulnerable study participants that are mainly comprised of incapacitated children and adults; they have to very communicative with such people so that information can be provided in a manner that is easy to understand. But researchers have experienced that they are unable to directly contact potential participants and required to make negotiations for accessing them through different gatekeepers. In areas where research has to be conducted by accessing people in organizational settings such as colleges in schools as well as social care settings, access to such people have to be negotiated by groups or individuals that manage such institutions. Agreements have to be made after meaningful process of negotiation to ascertain the manner in which the research participants are communicated with and asked to participate in the research study. It is known that there are different levels of gatekeepers for performing such functions and include professionals that administer organizations in which people can be accessed with the help of the guardians of patients. Although such gatekeepers are not vested with any legal rights in regard to people’s decision of participating in research activities they usually are in full control of the places where people are easily accessible. Additionally, these gatekeepers also have legal responsibility for people’s well-being in such settings. These circumstances imply that gatekeepers have the authority to refuse giving permission for researchers to use specific participants in any given setting. Moreover, in situations where approval is granted, gatekeepers have the authority to ascertain the manner in which the sort participants are given information about the study that is to be carried out in terms of their consent. This could possibly intact the willingness of participants to take part in research activity (Nuffield Council on Bioethics, 2002).
Introduction to Methodology
Research in generic means search for knowledge. Therefore, research methodology can be identified as a systematic approach adopted for solving a research problem scientifically, wherein the various steps are applied and analysed through logic means. The research methods are classified into 4 groups viz., (i) Descriptive vs. Analytical, (ii) Applied vs. Fundamental, (iii) Conceptual vs. Empirical and (iv) Quantitative vs. Qualitative. Here, quantitative research is applied with respect to the quantity of the data whereas, qualitative research, more or less, involves the quality or kind (Research Methodology, 2010).
According to Habermas (1972), every research made shall be in accordance with motivation which is guided by interests and values. The social and personal notions of the researcher will naturally find a place in such researches. Therefore, the value problems that may come up while the researcher traverses to fulfill the task will naturally become the guiding principles for the study. This research paper, as such, engages in ethical values and interests, ethical codes, legal implications, practices, societal welfare, privacy of individuals, legal protection to confidentiality of information etc. relating informed consent in health care. To complete the task the researcher has investigated the available literature and learning procedures which put insights to formulate ideas to achieve the objectives. Qualitative analysis and cross studies were incorporated to streamline the research.
The useable literature on informed consent in health care was re-examined and a questionnaire was contrived and utilized to gather applicable data for this research. Further, to find the required answer to the research questions, one to one interview was also held. The sampling population was selected in a purposeful way and qualitatively examined. There were some useful sources over the Internet about research methods which were put to study (Cronje, 2005; Trochim, 1996-2004).
Yin (1994) and STORRE (2002-2006) state that:
Every type of empirical research has implicit, if not explicit, research design. In the most elementary sense, the design is a logical sequence that connects empirical data to a study’s initial research questions and ultimately, to its conclusions. In a sense the research design is a blueprint of research, dealing with at least four problems: what questions to study, what data are relevant, what data to collect and how to analyze the results (Yin, 1994 & STORRE, 2002 – 2006).
Selection of research technique
There are many elements to be considered before selecting a method as there are benefits as well as drawbacks with every method chosen, and the researchers have to discover the most appropriate for them. According to Jarvinen (1999) it is more effective to separate research into approaches rather than methods. In addition, the research techniques utilized in the theory-testing approach are field studies, reviews, and field tries out. The features of such a study are the hypothesis, form or structure which is gathered from literature, formulated or polished to conform to that specific study. The purpose of this research was to find answers to the questions: (1) what are the ethical and legal implications of informed consent in healthcare? And (2) Will the present informed consent justify the elements of voluntary, competency and confidentially in patient-physician relationship?
The answers to these questions will give an insight to the pros and cons of the ethical and legal aspects of informed consent. Moreover, it will help to find out new measures to allow more confidentiality to the information provided by an individual in informed consent while availing legal rights to protection of one’s privacy. Moreover, it will help the healthcare policy makers to seek new methods to create awareness among the patients about the details provided by them in the inform consent and the necessities in filling up the form of informed consent. The main objectives of this research are:
- To conduct secondary research to establish the present ethics and legal rights inherent in informed consent
- To examine the contributions of healthcare institutions in observing the well being and privacy of the patients.
- To identify the potential problems in informed consent and to find solution to the problems to safeguard the interest of the patients at both the ethical and legal levels
- To find out whether the participants/patients are aware of the details of the content, status, function, and why there is need of the submission of written consent
When an investigator wants to construe an incident the study is expected to serve an understanding purpose. Firstly, the understanding should support the researcher to augment the knowledge of the studied incident. Secondly, it should build up new thoughts and theoretical views, and eventually the purpose permits disclosing of on hand problems inside the phenomenon. The present research is fulfilled by examining the views of professionals and individuals on the ethical and legal implications in informed consent in health care and cross studies of the acquired literature. Their opinions and studies were analysed to ascertain the feasibility and adaptability of their views and to know how far the outcome of such studies, suggestions and applications on informed consent infringe the privacy of the individuals either as research participants or patients who takes medical care. Additionally, it will provide status of understanding of the patients about the contents of the informed consent. The trend and the conviction of the public on the informed consent in the health care sector were collected systematically. While gathering data from these samples maximum effort was put in to avoid any embarrassment to the respondents and interviewees (Roberts & Patel, n.d.).
In order to ascertain the patients’ awareness about the legal implications of informed consent and their opinions on the function and submission of the form of consent questionnaires were issued to 732 patients who have undergone surgery in gynecology and obstetrics within 30 days of surgery. The setting for this study was fixed at a large teaching hospital (Akkad et al., 2006). Another survey was designed in the form of population-based questionnaire survey and conducted among 1410 people including men and women of 57 to 58 years of age. The questionnaire was given at random to gather data to know whether informed consent is necessary within the purview of ethical and legal codes in biomedical and health care research involving human participants (Länsimies-Antikainen et al., 2010).
A third study was conducted to see whether informed consent is an ethical and legal requirement in carrying out researches involving human participants. To achieve this consent was taken from parents of participants. They were asked to fill up a questionnaire containing questions about the elements of informed consent such as voluntary participation, privacy and confidentiality, risks and benefits of giving consent etc. The setting was placed in the environment of a developing country and 192 trial participants were selected for the purpose of the study (Minnies et al., 2008).
In addition to the questionnaire surveys mentioned above a set of nine predetermined questions was drafted for interviewing 32 patients having metabolic syndrome who were undergoing a medical experiment conducted for the evaluation of the effects of betaine in the case of cardiovascular risk factors. The interviews with these voluntary adult participants were meant to analyse and evaluate the application of informed consent in clinical researches (Lansimies-Antikainen et al., 2007)
The in depth one to one interviews and the questionnaire surveys helped the researcher much in exploring the topic of ethical and legal implications of informed consent in health care in detail. The interviews were short and objective, and were limited to duration of 15 minutes. The time of the interview was limited to 15 minutes to maintain the focusing on the questions and to avoid their unnecessary harangue. Such a limitation enabled the interviewees to maintain more interest in answering the questions with enthusiasm. Moreover, it helped the interviewees to save their much valued time.
Population and Sample
The researcher decides and describes the population based on the types of data that are collected with reference to time, territory and other appropriate component such as individuals and age. Furthermore, the region of the population has to be defined, and according to Miller, the territory is government limits e.g. a city or a region (Miller, 1998). Non-probability sample distribution is undertaken when the investigator has some control over the chosen sample. The investigator can select the sample established on judgment or repute, expediency, share, and/or offers (Miller, 1998). The questionnaire was therefore, given to the general public whose age was between 18 and 58 years old. I found that the people of this age group were more prudent in assessing a situation and were capable of answering the problems effectively. The questionnaire was also given to the medical professionals and service providers after obtaining permission from the hospital management, so that the answers would be very authentic and free from prejudices. The researcher was under the impression that they would interact with each other to share their thoughts about informed consent. The health care organizations which were selected for participating in the survey had more than 100 staff. The interviewees told the researcher that they have concern over the ethical and legal implications involved in the documentation relating to health care especially the informed consent, but they wanted to remain anonymous in this research. So their details were held back due to the researcher’s commitment to them that every detail they provided would be held confidential.
Data collection
The primary data collection was based on literature, interviews and questionnaire. The details provided by the informants were considered as supplementary data and therefore, the statistics and opinions given by the professionals and individuals were included in the study. Electronic archival research comprised of information from the webs and hard and soft copies of journals and other related sources. The interviews have become conducive to comprehensive analysis to which 32 individuals of varied ages were interrogated on the issues related to informed consent and their applications, advantages and disadvantages of such consents.
According to Daymon (2002), “there are no general set of laws and stages of qualitative data analysis; nevertheless the qualitative analysis is about reduction and interpretation”. The collected data were detailed and therefore, it was important to make them more manageable. The questionnaires were used to collect parts of the data and to analyse the results in a qualitative way. The results were examined and then a meaningful conclusion was drawn out of it. The analysis of the data which were collected through interview and questionnaire was done by means of inductive approach, wherein inductive analysis denotes the origination of information inherent in the data itself. The data thus collected were condensed and evaluated by adhering to the thematic codes. The themes were the resultant elements of the data, interview questions, questionnaire, and the learning of the available literature (Roberts & Patel, n.d.).
Questionnaire survey
Patients’ perceptions of written consent
The main purpose of the consent to treatment is to protect the autonomy of the patients and the Department of Health in England has issued formal guidance in getting the consent signed and the same is applied at all levels of treatment. But still, there lack thorough information about the understanding of the consent process. Most of them think that it is nothing but a ritual and are frightened to sign the written consent. Therefore, in order to ascertain the patients’ awareness regarding the legal implications of informed consent and their views on the functioning and submitting of the form of consent, pre-drafted questionnaires were posted to 732 patients who have undergone surgery in gynaecology and obstetrics within 30 days of surgery. The setting for this study was fixed at a large teaching hospital (Akkad et al., 2006). The findings of this survey were analysed and their results are given in the tables 3 to 5 below:
Perception on written consent: questionnaire study (Akkad et al., 2006).
Table 3 (Akkad et al., 2006)
In the above table is given the figures relating to the patients’ awareness of the legal implications in signing or not signing the consent form. Figures denote the numbers of women showing 95% confidence intervals (Akkad et al., 2006).
34% of responses were unsure whether the operation could be executed if they refuse to sign the form whereas, 17% believed it would be performed without signing the consent form. About 23% was unaware of the performing of the operation if they fail to sign though the non-intervention could prove fatal. 8% have wrong notions stated negatively. Most of the patients i.e. about 71% expressed ignorance about the fact that their next of kin were not entitled to sign on their behalf in the event of inability to sign it themselves (Akkad et al., 2006).
Table 4 (Akkad et al., 2006)
Views of the patients about the scope of consent forms: Figures denote the numbers of women showing 95% confidence intervals (Akkad et al., 2006).
Table 5 (Akkad et al., 2006)
Agreement of the patients regarding statements on the value and function of consent form. Figures denote the numbers of women showing 95% confidence intervals (Akkad et al., 2006).
Scope of consent
With reference to the table -3 above it is seen that 10% patients are unaware about what they have agreed to in the consent. The table-5 shows that 56% of the patients hold the belief that the doctor can adopt a different treatment procedure from the consented one if needed to save the life of the patient. 41% hold the view that the consent form is meant for knowing the likings of the patients. However, from table 2 it is evident that 86% know that their signatures sanctify their awareness that there involve some sort of risks in having the surgery (Akkad et al., 2006).
Value & function of the written consent
Table-5 inadvertently testifies that 70% of the patients have firm view that the consent was very important, whereas, 12% considered it as only a mere paper work. 40% signed it as they wanted the operation while 46% participants believed that the purpose of the consent was a step to safeguard the hospital from legal proceedings. However, 68% were satisfied that the signing of the consent form will enable the doctor to have control over whatever happens during the treatment procedure. On further analysis it reveals that 71% has accepted the fact that the signing of the consent form denoted the ensuing procedures relating to the treatment, and 77% knew that the consent would affirm the acceptance of the risks involved in the operation. But 36% viewed it as a precaution to safeguard the doctors and other staff of the hospital from any mishap in the operation theatre (Akkad et al., 2006).
The above analysis proves that though the patients need their legal rights in hospitals, their overall knowledge about the legal and ethical implications relating to the signing of the consent form is very limited and that while it fulfills the requirements of administrative and legal aspects, it does not meet the interests of the patients. Though there are advantages for the patients in signing the consent, there exist uncertainties about its performance such as execution of surgery without the consent, rights relating to compensation and validity of the proxy consent. There also exists lack of knowledge about rights to exercise the legal rights and awareness about the limits of the consent.
Evaluation of informed consent in health research
A second survey in the form of population-based questionnaire was conducted among 1410 people including men and women of 57 to 58 years of age. The questionnaire was given at random to gather data to know whether informed consent is necessary within the purview of ethical and legal codes in biomedical and health care research involving human participants. 91% of the participants have responded and the results showed that the basis of the informed consent is information as well as understanding together with competence and ability to voluntary decision making. However, there is still a minority who are not satisfied with the information received or follow the information provided (Länsimies-Antikainen et al., 2010).
Evaluation of the quality of informed consent in a developing country setting: (Minnies et al., 2008).
The procedure of Informed consent is a primary requirement ethically and legally where human participants are involved in the research. Guidelines regarding this are stipulated in the Declaration of Helsinki, the Belmont Report and Nuremburg Code. The International Convention for Harmonization ha taken initiative in the matter of Good Clinical Practice (GCP) and it is accepted as the quality standard for conducting clinical trials in human participants. Though research ethics in every country is regulated by relevant statutory bodies unethical research practices frequently occur. The third study is included in this dissertation in view of this situation. The purpose of this study is to assess the quality of consent and to identify influencing factors. Consent was collected from the trial participants’ mothers and they were asked to fill up the questionnaire and success in the selection of answers and interpretation of statements were measured. Informed consent was obtained in the mother tongue (Minnies et al., 2008).
Most of the 192 subjects interviewed obtained scores above 75% in recall as well as understanding sections. 79% of the participants knew the risks contained while 64% of the participants have exercised the decisions quite voluntarily. It is interesting to note that most of the participants have acquired scores above 75% for understanding. In recall it was 66% and it shows that the overall quality was progressive. The analysis on the understanding assessment reveals that low educational standard is not at all criterion in the participant’s capability in understanding the concepts of informed consent. These results can be counted as quality standard for public health research in a developing country (Minnies et al., 2008).
Recall of questions: Out of 9 recall questions four brought out 75% correct answers while the next four elicited 50% to 75% correct answers. The question for testing the participant’s knowledge about why they made the clinic visit provided the maximum correct answers i.e. about 85.4% (Figure-1). It is encouraging to note that only 36.5% of the participants were aware that the visits had no sudden benefits (Minnies et al., 2008).
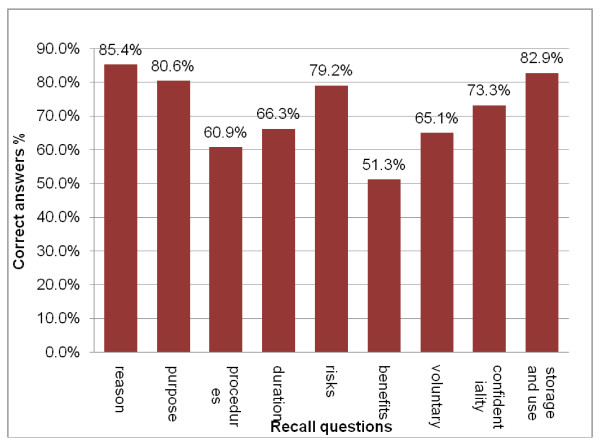
Over all results of the recall test: The percentage of participants who provided the correct answer to each of the questions (Minnies et al., 2008).
Participants who got Grade 7 and above at school obtained 75% and above in the recall test while the participants who were less educated scored less than 75%. Likewise, consent collected by nurses having research experience of above 2 years scored 75% against those having less experience. Understanding of questions: Most of the participants provided the correct answers for understanding questions. 87.5% realized that a bruise would not be a sufficient reason to seek legal rights. 66% believed that they could discuss the bruise with the nurse. From the logistic regression model the understanding scores obtained were 75%. It was also found that the older the participants the higher the scores. However level of education has nothing to do with the understanding (Minnies et al., 2008).
Most of the participants have knowledge about their rights relating to the health care, freedom in selection and also freedom from any danger (Figure-2). But they have answered wrongly about confidentiality and health care. Individual scores against health rights were not associated with recall scores while awareness of right to freedom of selection was correlated with understanding score. Overall, the study has revealed that quality of informed consent was directly associated education level and experience of the clinical professional who acquires the consent (Minnies et al., 2008).
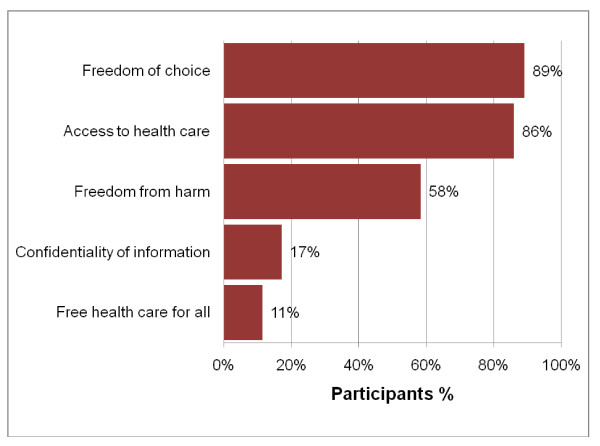
Knowledge of health care rights: The percentage of participants who provided the correct answers to the health care questions (Minnies et al., 2008).
Interview: Questionnaire
This interview was conducted among 32 patients having metabolic syndrome to analyse the application of informed consent in clinical researches. They were actually participating in another research to evaluate the effects of betaine on cardiovascular risk factor. Since informed consent is the basic principles of ethical research there is a further need to understand the implications of informed consent relating to the participants of research projects. Before the interview a set of 9 questions were served to them to gather required data. And during the interviews majority of the participants (81%) stated that the central elements of informed consent were information, voluntary decision and understanding. They were of the view that competency determines the reception of the information with proper understanding.
Questionnaire for the Interview: One-on-One Interview sample Questions (32) interviewees
This interview is strictly academic and the information provided will be kept confidential and will not be used against the interviewee or transmitted to a third person.
Gender: Male/Female (Please tick your gender)
Age: Above 18
- Have you participated in any other health care during the past 10 years?
Answer: Yes/No - Do you have any knowledge about informed consent?
Answer: Yes/No - Do you think that Informed consent is necessary in medical research?
Answer: Yes/No - Do you feel the present informed consent observes the ethical rights by the physicians or researchers?
Answer: Yes/No - Do you feel the present informed consent provides legal rights to the research participants?
Answer: Yes/No - Have you signed an informed consent earlier?
Answer: Yes/No - Do you think that present form of informed consent allows you to take decision voluntarily?
Answer: Yes/No - Do you think that present form of informed consent allows you to exercise your competency?
Answer: Yes/No - Do you feel that there are ethical and legal implications in the current informed consent?
Answer: Yes/No
Discussion
Informed consent is not a mere process of signing a document; it is more than signing a consent form by a patient. It is actually an important communication between patients and the doctor. From the various information provided in the literature review it is quite evident that it is an important document that has got ethical and legal implications concerned with health care applications. In the matter of patient-physician relationship, the way a doctor communicates with the patients is of prime importance. The pros and cons of the informed consent document signed by the patient depend upon the style of communication that is established by both the parties before commencing a surgical procedure on the patient.
It is true that obtaining a fully informed consent signed by the patient while taking a health care service is a very important good medical practice. It must be known that there are no ethical, legal and moral challenges that can be put against such a document except when the patient lacks competency and mental derailment or incapacitated otherwise. A finely executed informed consent will not invite litigations in the general sense and the outcomes of the process will be an advanced one without any complication and at the same benefitting the patient with a diminished rate in hospital expenses, shortened hospital stays and enhanced health care, and ultimately resulting in a win-win situation for both the parties. However, the problems facing such an execution of informed consent are innumerable. The main hurdles to it are communication gap, lacunae in understanding, ulterior motives of the physician or the healthcare management, lack of interest on the part of the patient, unethical attitudes of the health care professional, moral and cultural shortcomings of the service providers, ignorance on legal and ethical elements involved in the act, insincere and egoistic approach of the doctor and nurses, and non compliance to the standing statutes and regulations on health care. In addition to these are the situations of urgency to provide the life saving treatment to the patient, the unwanted interference of the relatives of the patient when a mishap occurs and the excessive time taken for the making of a signed informed consent. These elements contribute much towards obstacles in obtaining a proper and well informed consent. It is relevant to note that in case there occur some problems in the treatment ensuing litigation, the first thing the lawyer looks for is the contents of the very informed consent. If it is well maintained and obtained after fully informed to the patient the physician will never get headaches over this (Edwards, 1998).
The physicians should not go out of their way to seek fraudulent ways to obtain the signed informed consent from the patient prior to the treatment. Various studies and researches done by great visionaries and men of ethics and law concerned with health care have addressed the issues in several ways and their findings denote that there involve ethical and legal implications in the informed consent provided that it is not obtained in a sanctified way wherein sincere cooperation and harmony between the physician and the patient are the magic wand to document a good signed informed consent from the patient. The most important principle behind an informed consent is the interest to provide the opportunity to make choice to the patient and to make the patient avail of this opportunity to decide upon the choice of treatment the patient needs and the same shall be done for the common good.
As stated in the literature review by Kuper (2006); Wang (2000); and several other authors, a proper informed consent will have the following elements:
- the nature of the decision taken by the patient/procedure of healthcare
- reasonable options given to decide upon the proposed intervention
- the information about the relevant risks, benefits, and also the clues towards the information regarding the uncertainties ensuing each alternative
- the presence of assessment of patient’s understanding capacity
- the prompt acceptance of the intervention sought by the patient
For executing a valid informed consent the patients must be competent to make decisions by own and without any coercion from outside irrespective of whether it is the physician or the nurse or the standby. The physician should not utilize the convenience of patients’ vulnerability as they are powerless due to the illness. Thus the process of an informed consent is a fine document that invites the patients to undertake or participate in their health care decisions. However, the obligation of the physicians to share the reasoning process of the patient should be counted as a merit, provided they utilize the chance not to satisfy their ulterior motives, but to enhance their duty as pledged physicians where the duty is to extend service to the society (Edwards, 1998).
The statements of Lustig (1996); Asveld (2006); Maxwell J. Mohlman (2011); Macklin (2003) and several others that there are malpractices in obtaining consent from research participants are serious matters of concern to be dealt with by the policy makers of health care sector. Their observations regarding forceful participation of subjects in risk oriented health researches warrant litigation process. It is evident that the subjects can initiate legal proceedings in such cases. The ethical as well as legal implications involved in informed consent are more or less caused due to the lack of quality in the informed consent. A proper informed consent validates the purpose of the research and the participants’ leniency to it adhering to the belief that what they do favours the society at large. In that case their service to the society is great and the researchers should not take advantage of it. The meritorious informed consent that does not challenge the confidentiality of the participants when signed voluntarily will count for the satiation of high order community consensus. It will also result in the motive of the participants to extend their might to the welfare of the fellow people. Adherence to the ethical and legal components of the informed consent sanctifies the doctrine behind it.
While admitting the ethical and legal implications involved in informed consent it must be taken into consideration that the participants have the right to withdraw themselves from research participation and it is a fact that a part of the informed consent relates to allowing people the right to withdraw from their participation at any point of time. This also means that researchers need to ensure that they have in hand the ongoing consent of participants to take part in the given study. They also recognize that they are sensitive in the context of understanding the expressions and desire of participants to opt out of research studies.
The involvement of gatekeepers
There are two particular issues that have been identified in the context of gatekeepers and informed consent of participants in research activities. The first issue relates to excessive protection of gatekeepers that can lead to people who know how to take part in research activities but the opportunities are denied for them. The second issue is about the failure of gatekeepers in providing opportunity for possible participants in exercising their choices for taking part in research activities. In the context of the first issue there have been instances where gatekeepers have been denied access to researchers for a wide variety of reasons, such as assumptions of lack of competency levels amongst research participants. There are instances where access is agreed upon, gatekeepers are known to ask for consent from family members along with the consent of the concerned individual, especially in the context of children. This mostly implies that even if people wish to take part in research activities their consent maybe overruled by refusals by relatives. The issues of asking for parental consent in focus on sensitive matters such as sexual behaviors and drug use. Researchers are not able to force the organizations not to deny access and to ask for added consent from family members. The second issue of not getting potential research participants to assert their choice exists mostly in organizational settings and in school environments (Nuffield Council on Bioethics, 2002).
Apart from this, it is relevant to note that a well informed consent demands the following elements:
- Reasonable physician standard: This will enable the physician to decide upon what information is reasonable to be disclosed.
- Reasonable patient standard: This denotes the type and amount of information that a patient or research participant needs to understand the option available.
- Subjective standard: This component is somewhat challenging for the health care professional or researcher to maintain the standard of information that has to be provided to the patient or research participant (Edwards, 1998).
Most of the states in USA have legislation to determine the quality and standard of the informed consent. An informed consent can be treated as standard with quality if it is executed observing the following guidelines while discussing and disclosing information with the patients.
- Reveal the diagnosis about the patient, if known
- Reveal the nature and aim of the suggested treatment
- Disclose the risks as well as the benefits of the proposed treatment
- Discuss about the alternatives in hand regardless of the cost involved in it
- Discuss about the risks as well as the benefits involved in the alternative treatment
- Reveal the risks as well as the benefits regarding not availing a particular treatment or procedure.
Additionally, create a situation wherein the patient has an opportunity to ask the physicians certain questions to develop a better understanding on the position and to make a well informed consent either to proceed with the proposed new one or to refuse the medical intervention. This communication between the physician and the patient fulfills the ethical obligation and at the same time it will satiate the legal requirements (American Medical Association, 2011). Almost all of the researchers in biomedicine, public health sector and social-behavioural sciences face some challenges in adhering to the international regulations on informed consent under different socio-cultural environments. To avoid such problems there should be increased awareness about the ethical factors associated with their design of studies and the informed consent required to be obtained by the researchers while they work in the poorly resourced environment and settings. Moreover, empirical researches on different issues relating to the ethical and legal guidelines are required along with the capacity to cooperate with local communities to ensure that the outcome of the researches will be integrated into the concerned health system of those communities (Marshall, n.d.).
Summary, Conclusion and Recommendation
In terms of the objectives of informed consent, the most significant fact is that the patient is allowed the opportunity to become an informed participant while taking health care decisions. Mostly, it is accepted that informed concept in its entirety implies a discussion with the patient in relation to the nature of decisions and procedures as well as reasonable alternative. It also includes mention of relevant uncertainties, benefits and risks in the context of every available alternative. An important aspect of informed consent is making assessments of patient’s understanding and the acceptance of the given interventions by the patient.
Informed consent, in the context of readability also assumes significance in the comprehension and effectiveness of the patient. Most formats of informed consent are known to be very difficult for ordinary people to understand. According to the US census, almost 15 million people that live in urban areas do not have the ability to understand the English language effectively. Moreover, the health care system in the United States is known to make use of his adequate interpretation systems in healthcare. Healthcare facilities are known to make use of ad hoc interpreters such as employees, friends, family members and even strangers available in the waiting room. They are often called upon to explain the meaning and interpretation of medical instructions to patients. It is well established that such integrators often commit serious substitution or omission errors that could result in changing the meaning adversely. Moreover the patient’s family members or friends may not be able to explain all the details of the proposed medical intervention in the belief that the omission will be in the betterment of the patient.
The circumstances relating to cultural and information disparities impact upon the understanding of the patients because they usually expect to receive information regarding the proposed surgical treatment passively. After being instructed before one-time readings they are made to sign the consent form without obtaining copies for themselves. The wording is in the consent form which is usually some technical terms or medical terms thereby decreasing the understanding level of patients. Such circumstances also increase the chances that the patient may feel inclined to take legal action against physicians.
In the legal perspective, competency has been defined rather vaguely by courts but certain levels of standards to prevail in the context of determining informed consent and signing on them. The legal practitioners and nurses have to attain more knowledge in the context of laws in different states in the context of competency. Aged patients would be suffering from hearing impairment, slow patterns of thinking and the presence of transient confusion. Many aged patients suffering from stroke often suffer from difficulties in speaking and thus require additional time for effective communication. Patients often refuse to give answers to questions because they are not satisfied about being in hospital. Though any of such issues can be involved, if patients are encouraged to paraphrase what they understand about the given processes it proves to be the most effective way in determining whether they are aware of the provided information. Thus patients are able to understand the benefits and associated risks. However, such factors do not necessarily imply that there is no competence or decision making capability amongst health care professionals. Under situations when the capacities of patients are doubted by medical practitioners, they usually obtain informed consent from the nearest relatives. It is obvious that assigned informed consent by the patients of collective is a better option as compared to not having any consent at all.
For informed consent to be entirely valid patient has to meet the given conditions so that he is legally competed to make the given decisions. Informed consent has to be voluntary because it is quite usual for coercive circumstances to surface in medical treatment which will make the patients feel vulnerable and powerless in the given situations. In order to encourage the sense of involuntary decision the physicians are required to convince the patients that they are participating in decisions and not just signing a document. Given this understanding the process of informed consent is viewed as an invitation to patients to take part in their health care decisions.
The physician also has an obligation to make appropriate recommendations and to share his or her opinions with the patient in regard to the given procedures. It is also important for the patient to comprehend the given information which requires the discussion to be conducted in a manner that a normal individual understands the terms and conditions and can make proper assessments along the way. Basically informed consent allows patients to know what they like to do by asking them if the given procedures are acceptable to them.
Recent developments in medical research have raised a number of issues about the efficiency and effectiveness of government regulations governing the manner in which medical research is conducted in all aspects of clinical trials and surgical and medical treatments and research. The legal definitions of human subject and private information that have been governing the course and practices adopted by research for the last two decades are currently being analyzed in view of the ambiguity due to wrong methods and practices. Researchers are known to customarily call for information relating to family history in order to track and characterize genes and cells of patients belonging to larger families. Piling up family history information mostly requires researchers to ask patients and subjects about the health conditions of collateral family members.
The issue arises if these family members should be treated as subjects for the purpose of providing informed consent. Such issues have led to considerable debate and a large scale exercise to re-examine the present federal regulations and rules regarding informed consent. Future practices and regulation in the context of informed consent should make a review of the developments made in the present federal regulations and the extent to which they provide a basis and a means for departing from the issue to resolve the present issues in medical research. Secondly, a review should be made of the usual practices in medical research as it will further the regulation and guidelines for addressing the given issues in medical research. Thirdly, it is also required that all new regulations and guidelines should enable proper protection to individuals and their family members who are taking part in the research exercises while giving support to related activities in medical research.
Obtaining informed consent from potential research subjects is obviously not a straightforward procedure. Researchers are required to a concentration to a wide range of matters while providing information to research participants and while getting their informed consent. Such issues are inclusive of the styles, formats and timing of information. The environment in which the research is carried out and the ethical orientation of research studies have a strong impact on the approach adopted by researchers in such issues. But the decision about informed consent is largely driven by regulatory, ethical and legal frameworks in which the research is carried out. A number of changes have taken place in terms of ethical regulation in the medical and social research which evolved consequent to a wide variety of moral, political, legal and institutional influences, which means that such issues are likely to change in the near future.
References
Ananworanich J et al. 2004. Creation of a drug fund for post-clinical trial access to antiretrovirals. Lancet, 101–102.
Angell M. 2006. Investigators’ responsibilities for human subjects in developing ountries. New England Journal of Medicine, 342:967–969.
Appelbaum PS, Roth I, Lidz C. 2002.The therapeutic misconception: informed consent in psychiatric research. International Journal of Law and Psychiatry, 5:319–329.
Atkison, J. 2004. Private and Public Protection. Civil Mental Health Legislation. Edinburgh. Dunedin Academic Press.
Baker R. 2006. A theory of international bioethics: the negotiable and the non- negotiable. Kennedy Institute of Ethics journal, 9:234–274.
Berg, Jessica W., Paul S. Appelbaume, Lisa S. Parker, and Charles W. Lidz. 2003. Informed Consent: Legal Theory and Clinical Practice. Oxford University Press.
Beauchamp, T. L, and Childress J. S, (2001) Principles of Biomedical Ethics. Oxford: Oxford University Press.
Benedetto V, Petert SJ. 2005. Medication development and testing in children and adolescents. Arch Gen Psychiatry, 54 : 871-6.
Boris B, David A. 2005. Pediatric psychopharmacology. In: Benjamin JS, Virginia AS, editors. Comprehensive text book of psychiatry. 8th ed. Philadelphia: Lippincott Williams & Wilkins. pp. 3363-76.
Brody, B. A. 2004. The Ethics of Biomedical Research. Oxford: Oxford University Press.
Capron A. M, 2006. Ethical and Human-Rights Issues in Research on Mental Disorders that may Affect Decision-Making Capacity. In: De Grazia D, and Mappes T. A (eds.). Biomedical Ethics. New York: McGraw Hill: pp. 251-257.
Collste, G. 2007. Applied and professional ethics – an introdution. In: Collste, G (ed.), Perspectives on Applied Ethics (Centre for Applied Ethics: Linkopings universitet, Sweden).
Druml C. 2004;10.6:570-3. Informed consent of incapable ICP.patients in Europe: existing laws and the EU Directive, Curr Opin Crit Care.
Dworkin G, (2005) Paternalism. In: Zalta E. N (ed.). The Stanford Encyclopedia of Philosophy. Web.
Fisher.JA., 2006. Procedural Misconceptions and Informed Consent: Insights from Empirical Research on the Clinical Trials Industry, Kennedy Institute of Ethics Journal; 16.3: 251-268.
Freemon FR. 2004. Gangrene and Glory: Medical Care During the American Civil War. Champaign, Ill: University of Illinois Press.
Harvey AM. 2003. John Shaw Billings: forgotten hero of American medicine. Perspect Biol Med. 21;36–57.
Health Research (2006) World Health Organization.
Henderson GE, Stein J, eds, 2006. Beyond regulations: ethics in human subjects research. Chapel Hill: University of North Carolina Press, 214–224.
Holmes-Rovner M. 2002 Wills CE. Improving informed consent: insights from behavioral decision research. Med Care. Suppl.V30-8.
Hyder AA, Wali SA. 2006. Informed consent and collaborative research: perspectives from the developingb world. Developing World Bioethics, 6:34-40.
Jsselmuiden CB, Faden RR. 2004. Research and informed consent in Africa—another look. New England Journal of Medicine, 326:831–833.
Indian Council of Medical Research. 2004. Ethical guidelines for biomedical research on human subjects. New Delhi, India.
Institute of Medicine. 2005. Responsible research: a systems approach to protecting research participants. Washington, DC: National Academies Press.
Jinadu MK et al. 2007. Partnership for primary care. World Health Forum, 18:212–214.
Jones J. 2003. Bad blood: the Tuskegee syphilis experiment. New York: Free Press.
Joubert G et al. 2005. Consent for participation in the Bloemfontein vitamin A trial: how informed and voluntary? American Journal of Public Health, 93:583–584.
Juengst ET. 2004. What ‘community review’ can and cannot do. Journal of Law, Medicine and Ethics, 28:52–54.
Katz, R. V. et al. 2009. Identifying the Tuskegee Syphilis Study: implications of results from recall and recognition questions. In: BMC Public Health, 9:468. Web.
Kaufert JM, O’Neil JD. 2005. Biomedical rituals and informed consent: native Canadians and the negotiation of clinical trust. Social science perspectives on medical ethics. Philadelphia: University of Pennsylvania Press, 40–64.
Kaufert JM, Putsch RW. 2006. Communication through interpreters in healthcare: ethical dilemmas arising from differences in class, culture, language, and power. Journal of Clinical Ethics, 8:72–87.
Kaufert JM, Putsch RW, Lavallee M. 2004. Experience of aboriginal health interpreters in mediation of conflicting values in end-of-life decision making. International Journal of Circumpolar Health, 57( 1):44–48.
Katz J. 2003. Experimentation with human beings. New York: Russell Sage Foundation.
Kuczewski M, Marshall P. 2004. Decision dynamics in clinical research: the context and process of informed consent. Medical Care, 40:42–54.
Kuper A. 2006. Culture: the anthropologists’ account. Cambridge: Harvard University Press.
Lavery JL. 2003. Putting international research ethics guidelines to work for the benefit of developing countries. Yale Journal of Health Policy,2006. Law and Ethics, 3:318–336.
Leach A et al. 2005. An evaluation of the informed consent procedure used during a trial of a Haemophilus influenzae type B conjugate vaccine undertaken in The Gambia, West Africa. Social Science & Medicine, 48:139–148.
Lock M, Gordon D. 2007. Biomedicine examined. Dordrecht, Netherlands: Kluwer Academic Publishers.
Ludmerer K. 2003. Time to Heal: American Medical Education from the Turn of the Century to the Era of Managed Care. New York, NY: Oxford University Press.
Macklin R. 2003. Against relativism: cultural diversity and the search for ethical universals in medicine. New York: Oxford University Press.
Mappes, T, and De Grazia, D. 2005. Biomedical Ethics, New York: Mc Graw Hill, Inc.
Marshall P. A. et al., 2006. Voluntary Participation and Informed Consent to International Genetic Research. American Journal of Public Health; 96.
Moreno J. D, et al. 2004. Informed Consent. In: Chadwick R. Encyclopedia of Applied Ethics 2. New York: Academic Press: 687-696.
Nuffield Council on Bioethics, 2002. The Ethics of Research Related to Healthcare in Developing Countries. London: Nuffield Council on Bioethics.
Walton N, 2010. What is research ethics? Web.
Wang C E. 2000. Ethical issues in medical malpractice, Volume 4:294-8. Sci Q.
World Medical Association: 2003. Declaration of Helsinki: Recommendations guiding physicians in biomedical research involving human subjects. 277 : 924-6.
Additional references
Akkad, A., Jackson, C., Kenyon, S., Woods, M.D., Taub, N. and Habiba, M. (2006) Patients’ perception of written consent: questionnaire study. BMJ; 333 : 528. Web.
American Medical Association (2011) Informed Consent. Patient Physician Relationship Topics. Web.
American Psychiatric Association (2007) Psychiatric Services Vol 58: Issue 2, American Psychiatric Publishing, Inc. Web.
Cappelen, A.W. and Norheim, O.F. (2005) Responsibility in health care: a liberal egalitarian approach. J Med Ethics;31:476-480. Web.
Delany, C.M. (2005) In private practice, informed consent is interpreted as providing explanations rather than offering choices: a qualitative study. Australian Journal of Physiotherapy 2007 Vol. 53. Web.
Edwards,A. (1998) Informed Consent, Ethics in Medicine: Project Bioethics Education Project , University of Washington School of Medicine. University of Washington. Web.
IJsselmuiden, Carel B. and Faden R. (1992) Research and informed consent in Africa—Another look. The New England Journal of Medicine 326(12):830–834.
Indian Council of Medical Research Bulletin (2004) Health Research Policy, Vol.34, No.9-10 ISSN 0377-4910. Web.
Jonsen, A.R., Veatch, R.M. and Walters, L.R. (1998) Source Book in Bioethics, A Documentary History ISBN: 9780878406852 LC: 9741521, Georgetown University Press, Washington DC.
Karim, A, Quarraisha, S. S., Karim, A, Coovadia, H.M. and Susser, M. (1998) “Informed consent for HIV testing in a South African hospital: Is it truly informed and truly voluntary?” American Journal of Public Health 88(4):637–640.
Kapp, M.B. (2006) Ethical and legal issues in research involving human subjects: do you want a piece of me? Journal of Clinical Pathology. 59:335-339. Web.
Kuther, T.L. (2003) Medical decision-making and minors: issues of consent and assent. Adolescence. Libra Publishers, Inc. Web.
Lansimies-Antikainen, H., Laitinen, T., Rauramaa, R. and Pietila, A.M. (2010) Evaluation of informed consent in health research: a questionnaire survey. Scandinavian Journal of Caring Sciences, 24: 56–64. Web.
Lansimies-Antikainen, H., Pietila, A. M., Laitinen, T., Schwab, U., Rauramaa, R. and Länsimies, E. (2007), Evaluation of informed consent: a pilot study. Journal of Advanced Nursing, 59: 146–154. Web.
Lee, W.H., Kim, I.S., Kinf, B.H., and Kim, S. (2008) Probing the Issue of Informed Consent in Health Care in Korea—Concept Analysis and Guideline Development. Asian Nursing Research. Web.
Lustig, B.A. (1996) Informed consent as a tool for medical management- The Journal of Medicine and Philosophy. 21:101-109, Kluwer Academic Publishers.Netherlands. Web.
Mallardi, V. (2005) The origin of informed consent. Acta Otorhinolaryngol Ital. National Center for Biotechnology Information, U.S. National Library of Medicine. 25(5):312-27. Web.
Marshall, P.A., (n.d.) Ethical challenges in study design and informed consent for health research in resource-poor settings, Special Programme for Research & Training in Tropical Diseases (TDR), World Health Orgnization, ISBN 978 92 4 156338 3. Web.
Melton, G. B. (1983). Decision making by children: Psychological risks and benefits. In G. B. Melton, G. P. Koocher, & M. J. Saks (Eds.), Children’s competence to consent (pp. 21-40). New York: Plenum
Mehlman, M.J. (2011) Informed Consent, Bioethics. Web.
Minnies, D., Hawkridge, T., Hanekom, W., Ehrlich, R., London, L. and Hussey, G. (2008) Evaluation of the quality of informed consent in a vaccine field trial in a developing country setting. BMC Medical Ethics, 9:15. Web.
Paterick T.J., Carson G.V., Allen, M.C., Paterick,T.E,. (2008) Medical informed consent: general considerations for physicians. Mayo Clin Proc. 83:313–9. Web.
Rainbow, C. (2002) Informed Consent, Department of Biology, Davidson College. Web.
Rosenfeld, B. (2002) The Psychology of Competence and Informed Consent: Understanding Decision-Making with Regard to Clinical Research. Fordham Urban Law Journal, Vol. 30. Web.
Tess, P. (1997) Legal and ethical considerations of informed consent (medical consent). AORN Journal. Web.
Veatch, R.M (1987). The patient as partner: a theory of human experimentation ethics. Bloomington: Indiana University Press.
Wood, S.Y., Friedland, B.A., McGrory, C.E. (2001) Informed Consent: From Good Intentions to Sound Practices, Population Council, New York, NY. Web.