Introduction
Background
According to the nomenclature suggested by the DSM-V, phobias are referred to as a specimen of anxiety disorders. Moreover, the specified phenomenon is often compared to the social communication disorders since most of the symptoms overlap (American Psychiatric Association, 2013). According to the commentary provided by the American Psychiatric Association, “in social anxiety disorder, the social communication skills developed appropriately but are not utilized because of anxiety, fear, or
distress about social interactions” (American Psychiatric Association, 2013, p. 49). In other words, the subject matter concerns primarily a temporary impairment of one’s ability to communicate properly.
When addressing the needs of the patients with the above condition, therapists typically consider a combination of therapies and medications. The use of Paxil as one of the most efficient tools in managing the disorder is typically approved (Parsons, 2015). However, the side effects of Paxil may nonetheless be rather heavy, including sleep disorders and restlessness as well as impotence in men (Sureka, Desai, & Gupta, 2013). Placebo is typically viewed as the alternative to the adoption of medication-based treatment (Kamaradova, Prasko, Sandoval, & Latalova, 2014).
Objectives
- Primary objective. The study aims at detecting whether the application of Paxil in people with phobias, in general, and social phobias (e.g., the fear of communicating with other people, the fear of voicing one’s opinion, etc.), in particular, is more efficient than the use of placebo when combined with therapy.
- Secondary objective. The research is also aimed at detecting the possible side effects of the use of Paxil as the mean of addressing the patients’ anxieties.
Hypothesis
It is suggested that the adoption of Paxil has a stronger impact on patients than the provision of placebo as the means of addressing their fears, although Paxil may also have adverse effects on the participants due to personal intolerance of the medication or its components.
Design and Methodology
Research Design
The study will be carried out with the help of a cross-over balanced placebo design trial (CBPD). The specified type of placebo train implies splitting the patients into two groups, i.e., Group A, in which the patients will be provided with the stated drug (i.e., Paxil) (a reduction from 20 mg to 10 mg per day in the course of two months) along with therapy, and the control group, which will consume placebo instead of Paxil, though also undergoing the therapy process. Throughout the trial, the patients’ safety will be provided by monitoring the effects of drug applications and identifying the instances of individual intolerability (Sinacola, 2015).
Participants
40 participants from a local hospital will be recruited to participate in the study.
Inclusion criteria
- The participant’s condition meets the DSM-V criteria for a phobia as a specimen of the anxiety disorder;
- The patient is aged 25-50;
- The patient is accompanied by a nurse or a companion.
Exclusion criteria
- Major physical impairments (e.g., vision, locomotor system, hearing, etc.);
- The existence of other psychiatric or medical conditions;
- Inability to give an informed consent;
- Intolerance of the identified medication (Paxil);
- MRI, EKG, blood pressure, or any other test findings indicating abnormal results.
Informed consent
The participants will have to sign an informed consent, therefore, agreeing to participate in the research.
Ethical Standards
The research will follow the current UK standards for cross-over balanced placebo design trials (CBPD). Seeing that the use of placebo in control groups may conflict with the current ethical standards accepted in healthcare (Enck, Klosterhalfen, Weimer, Horing, & Zipfel, 2011), the patients will be warned that they may appear in a group where they will be provided with placebo (Upadhyaya et al., 2013) as opposed to the actual medicine. In addition, the information regarding the costs of the project, the type of medicine used, the potential risks and benefits as well as possible threats to the patient’s wellbeing will be outlined in the Patient Information Sheet (PIS) (Gega et al., 2012).
Methodology
Data collection tools
The data will be collected with the help of the notes made in the course of patient observation (Goldin et al., 2013).
Data analysis tools
A two-way ANOVA test will be used to identify the correlation between the variables. Thus, the relation between the key variables (i.e., the application of Paxil in the instances of anxiety disorders, particularly, panic attacks and fears, the use of placebo, and the following improvement in the patients’ state) can be identified successfully and with high accuracy rates (Cuypers, Lamers, Kil, Poll-Franse, & Vries, 2015).
Sample size
The identification of the sample size is justified by the endpoint means comparison. In the specified research design, α=00.5, whereas the power is estimated at 80%. The formula suggested by Charan and Biswas (2013) was used to identify the actual sample size:
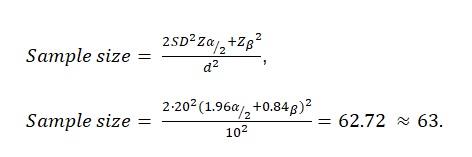
As the calculations above show, the sample size of the target population is 63 people. Therefore, 63 patients will be selected for the study.
Statistical analysis
In light of the fact that the study will encompass two groups, i.e., the target one (the patients provided with a reduced amount of Paxil along with regular therapies) and the control one (the patients treated with therapy and placebo), it will be reasonable to adopt a t-test (Schmid, McAlindon, Schmid, & Wang, 2013). Thus, the initial hypothesis will be verified. As far as the secondary objectives are concerned, it is recommended that a descriptive analysis should be adopted (Crowcour et al., 2012).
Cost estimations
As shown in Table 1 (see Appendix A), the project will require a total of £500,000. It could be argued, though, that costs can be reduced with the introduction of a sustainable approach (Larson et al., 2013) based on the use of a smaller number of staff members. However, to avoid the possibility of workplace burnouts among nurses (Yeun & Han, 2016), it is desirable to spend the identified amount of money.
Rationale
Design
The choice of the dose was predetermined partially by the financial restrictions. Particularly, the budget did not permit covering three powered arms, which meant that the drug had to be split into 30 mg and 20 mg arms correspondingly (Zhang et al., 2012). In addition, the adoption of the placebo-controlled trial was essential for the veracity of the research outcomes. The CBPD tool was used in the Paxil in AD efficacy trial seeing that it allows obtaining accurate results as its application in previous trials has shown (Cosgrove & Black, 2013), though minor hindrances can be expected (Lund, Vase, Petersen, Jensen, & Finnerup, 2014).
Pharmacodynamics
The transfer from the 30-mg (yellow) to 20-mg (pink) tablets in the course of the first week will be carried out among the target group of the patients (Bystritsky, Khalsa, Cameron, & Schiffman, 2013). Seeing that the mean elimination half-life in Paxil equals approximately 21 hours, it will be reasonable to restrict the drug intake to one tablet per day (Dobbs, 2013). Seeing that the drug is defined as an inhibitor of neuronal serotonin reuptake (Orak, 2015), patients with cardiovascular disorders should be excluded from the study as well (Pickham & Sickler, 2013).
Strengths
Seeing that the calculation of the sample size of the patients was carried out based on the outcomes of a t-test (Schmid et al., 2013), the further results of the study are bound to have a high accuracy rate. The precision, which the analysis will allow for, swill help locate the most efficient tools for addressing the subject matter and locate the usefulness of each treatment method (Roest et al., 2015). As a result, a significant increase in the possibility for a faster and a more efficient recovery of the patients will emerge (Tomita et al., 2014).
In addition, the adoption of the PIS principles as the foundation for the ethical framework of the research will prevent any possible ethical dilemmas from emerging in the designated environment (Chaudhry et al., 2013). It is essential that every single patient should be aware of the consequences and give a personal informed consent for participating in the trial. As a result, the possibility for obtaining more accurate results and the identification of the most efficient approaches in treating phobias and panic attacks as the manifestation of the anxiety disorder can emerge (Roest et al., 2015).
Limitations
It should be noted that some of the patients may develop resistance toward the specified drug (i.e., Paxil). Particularly, the overweight participants of the study as well as patients with obesity are likely not to be affected by the medicine under analysis. Therefore, the issue regarding patient safety needs to be addressed.
In addition, the effects of placebo will not become evident immediately. Instead, one will have to wait for approximately four months to identify the outcomes of placebo application, which means that the study may have to be prolonged.
Reference List
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Publishing.
Bystritsky, A., Khalsa, S. S., Cameron, M. E., & Schiffman, J. (2013). Current diagnosis and treatment of anxiety disorders. Pharmacology & Therapeutics, 38(1), 30-45.
Charan, J., & Biswas, T. (2013). How to calculate sample size for different study designs in medical research? Indian Journal of Psychological Medicine, 35(2), 121-126. Web.
Chaudhry, I., Husain, N., Husain, M. O., Hallak, J., Drake, R., Kazmi, A…. & Deakin, B. (2013). Ondansetron and simvastatin added to treatment as usual in patients with schizophrenia: study protocol for a randomized controlled trial. Trials, 14(1), 101-109. Web.
Cosgrove, S. D., & Black, K. E. (2013). Sodium supplementation has no effect on endurance performance during a cycling time-trial in cool conditions: a randomised cross-over trial. Journal of the International Society of Sports Nutrition, 10(1), 30-36. Web.
Crowcour, S., Leibing, E., Ginzburg, D., Stangier, U., Wiltink, J., & Hoyer, J. (2012). Transfer of manualized CBT for social phobia into clinical practice (SOPHO-PRAX): a study protocol for a cluster-randomized controlled trial. Trial, 13(1), 70-78. Web.
Cuypers, M. B., Lamers, R. E. D., Kil, P. J. M., Poll-Franse, L. V. v., & Vries, M. d. (2015). Impact of a web-based treatment decision aid for early-stage prostate cancer on shared decision-making and health outcomes: study protocol for a randomized controlled trial. Trial, 16(1), 231-240. Web.
Dobbs, A. S. (2013). A little better than placebo is still better than nothing . Nature Medicine, 19(8), 962.
Enck, P., Klosterhalfen, S., Weimer, K., Horing, B., & Zipfel, S. (2011). The placebo response in clinical trials: More questions than answers. Philosophical Transactions of the Royal Society, 366(1572), 1889–1895.
Gega, L., Swift, L., Barton, G., Todd, G., Reeve, N., Bird, K., Holland, R., Howe, A., Wilson, J., & Molle, J. (2012). Computerised therapy for depression with clinician vs. assistant and brief vs. extended phone support: study protocol for a randomised controlled trial. Trials, 13(1), 151-161. Web.
Goldin, P. R., Ziv, M., Jazaieri, H., Hahn, K., Heimberg, R., & Gross, J. J. (2013). Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs randomized clinical trial. JAMA Psychiatry, 70(10), 1048-1056. Web.
Kamaradova,D., Prasko, J., Sandoval, A., & Latalova, K. (2014). Therapeutic response to complex cognitive-behavioral and pharmacological treatment in patients with social phobia. Activitas Nervosa Superior Rediviva, 56(3–4), 91-99.
Larson, B. A., Bii, M., Henly-Thomas, S., McCoy, K., Sawe, F., Shaffer, D., & Rosen, S. (2013). ART treatment costs and retention in care in Kenya: a cohort study in three rural outpatient clinics. Journal of International AIDS Society, 16(18026), 15. Web.
Lund, K., Vase, L., Petersen, G. L., Jensen, T. S., & Finnerup, N. B. (2014). Randomised controlled trials may underestimate drug effects: Balanced placebo trial design. PLoS ONE, 9(1), e84104. Web.
Orak, Y. (2015). Serotonin syndrome due to overdose intake of SSRI. Case report. Journal of the Turkish Society of Intensive Care, 13(1), 79-82. Web.
Parsons, T. D. (2015). Virtual reality exposure therapy for anxiety and specific phobias. Psychology and Human Behavior, 1(1), 288-2296. Web.
Pickham, D., & Sickler, K. (2013). Woman with risks for torsades de pointes dying within hours of leaving the emergency department. Journal of Emergency Nursing, 39(1), 53-56. Web.
Roest, A. M., Jonge, P. d., Williams, C. D., Vries, Y. A. d.,. Schoevers, R. A., & Turner, E. H. (2015). Reporting bias in clinical trials investigating the efficacy of second-generation antidepressants in the treatment of anxiety disorders. JAMA Psychiatry, 72(5), 500-510. Web.
Schmid, A., McAlindon, T., Schmid, C. H., & Wang, C. (2013). The Influence of tai chi exercise on proprioception in patients with knee osteoarthritis: Results from a pilot randomized controlled trial. International Journal of Integrative Medicine, 1(1), 37-45.
Sinacola, R. S. (2015). Pharmacologic management of anxiety spectrum disorders. Audio-Digest Psychology, 4(1), 1-3.
Sureka, P., Desai, N., & Gupta, D. K. (2013). A study of subsyndromal and syndromal psychiatric morbidity among male patients with alcohol dependence. ASEAN Journal of Psychiatry, 14(2), 146-156.
Tomita, T., Norio, Y.-F., Sato, Y., Nakagami, T., Tsuchimine, S., Kaneda, A., & Kanek, S. (2014). Sex differences in the prediction of the effectiveness of paroxetine for patients with major depressive disorder identified using a receiver operating characteristic curve analysis for early response. Neuropsychiatric Disease and Treatment, 10, 599–606. Web.
Upadhyaya, H., Adler, L. A., Casas, M., Kutzelnigg, A., Williams, D., Tanaka, Y., Arsenault, J., Escobar, R., & Allen, A. J. (2013). Baseline characteristics of European and non-European adult patients with attention deficit hyperactivity disorder participating in a placebo-controlled, randomized treatment study with atomoxetine. Child and Adolescent Psychiatry and Mental Health, 7(1), 14-22. Web.
Yeun, Y.-R., & Han, J.-W. (2016). Effect of nurses’ organizational culture, workplace bullying and work burnout on turnover intention. International Journal of Bio-Science and Bio-Technology, 8(1), 372-380. Web.
Zhang, S., Kan, Q., Wen, J., Zhao, J., Sheng, Y., Li, Y…., & Lin, Z. J. (2012). A pilot study for a pivotal bioequivalence trial using two paroxetine 40 mg tablet formulations in healthy Chinese subjects. International Journal of Clinical Pharmacology and Therapeutics, 50(1), 33-43. Web.