Risk Assessment
What are the hazards and do they prevent ‘lone working’?
Sodium acetate buffer- It is an irritant that can destroy the integrity of the skin as well as the lining of the gastrointestinal tract. Sodium acetate can also irritate the eyes and affect the sense of sight. A spray mist of the chemical can lead to the formation of chemical vapour, which can be inhaled thereby leading to the damage of the mucous membranes found in the eyes, mouth and respiratory system.
Phosphate buffer- This chemical is dangerous when inhaled, swallowed or taken up by the skin. Phosphate buffer can also cause irritation to the eyes, skin or the mucous membranes that line the respiratory tract.
Sulphuric acid- This chemical is a highly corrosive substance that can cause severe burns when handled carelessly. However, the concentrated form of the acid is more dangerous than the dilute form. In this experiment, dilute sulphuric acid will be used as the stop solution. Two types of burns can arise from sulphuric acid: chemical and secondary thermal burns. A secondary thermal burn occurs from the heat that is produced when sulphuric acid makes contact with substances containing water (exothermic reaction). Sulphuric acid can “eat up” work surfaces and metal. Therefore, it can also destroy the work area. Permanent blindness can occur when this acid gets into the eyes. On the other hand, the accidental swallowing of the acid can cause irreversible damage to the gastrointestinal tract. High concentrations of sulphuric acid aerosol or vapour can have adverse effects on the respiratory system and the eyes. Long standing exposure to mild forms of the acid can wear down teeth.
The above-mentioned hazards cannot get in the way of lone working because they do not pose high-level risks. The experimental procedures as highlighted in the laboratory manual entail drawing small volumes of the solutions using automated micropipettes. Therefore, aerosols will not be formed, which will reduce the risk of inhalation. Automated micropipettes will eliminate the risk of accidental swallowing of the chemicals, which would happen with other types of pipettes. Furthermore, dilute sulphuric acid will be used as opposed to the concentrated form of the acid that is a high-level hazard.
What will be done in the procedure?
The working analytical chloramphenicol standards will be prepared in the assigned groups as indicated in the lab manual. The horseradish peroxidase (HRP) will be prepared by mixing 1/10000:400µl of 1/1000 HRP with 3600µl blocking buffer (sodium acetate) as well as 1/5000:800µl of 1/1000 HRP and 3200µl blocking buffer. The standards and unknown samples were then added to the wells followed by the HRP enzyme conjugate and incubated. At the end of the incubation period, the microtiter plates were washed by filling the wells with 300µl of the wash buffer and emptying them eight times. Thereafter, 100 µl of TMB-E will be added to each well and incubated for 12 min at 37°C. In the last step, 25 µl of 2.5M sulphuric acid stop solution will be added to each well. The absorbance of the plates will then be measured.
How will the risks be minimised?
Possible risks will be minimised by donning personal protective gear. Relevant examples include protective goggles, chemical resistant gloves and protective clothing. Shoe covers can also be considered to protect the feet in the event of accidental spillage.
Are there any specific risks to females of childbearing age who could become
pregnant or any risks to new and expectant mothers?
Women of childbearing age can be endangered through exposure to chemicals that interfere with the reproductive system. Conversely, pregnant women can be affected by chemicals that can affect the developing foetus. Carcinogens and mutagens can lead to birth defects in unborn babies. Currently, there are no reports regarding carcinogenic or mutagenic effects of the reported hazardous chemicals. Therefore, there are no specific risks for pregnant and females of childbearing age.
What is the assessed risk?
The assessed risk is small and is mostly linked to skin contact with chemical substances. The use of personal protective equipment will alleviate this risk.
Is health surveillance required?
There is no need for health surveillance because the chemicals and experimental steps cannot affect specified groups of people, including expectant females or women of childbearing age. In addition, adequate measures are in place to lessen the risk.
How will you dispose of the material used?
When disposing of laboratory materials, it is necessary to ensure that the waste does not pollute the environment or cause further chemical degradation. Therefore, waste materials used in the experiment will be disposed of according to their physical and chemical properties. At the end of the reaction, corrosive liquids such as sulphuric acid will have reacted with other components of the assay, leading to the formation of non-corrosive products. Therefore, these waste substances will be washed down the sinks using a lot of water. Unused dilute solutions will also be discarded in the same way. The used ELISA plate will the main solid waste, which will be disposed of in the solid waste bin.
Procedure in case of accident or spillage.
In the event of an accident or spillage, different measures will be undertaken based on the chemical nature of the substance involved. Spills involving corrosive substances will first be absorbed from surfaces using absorbent pads. Thereafter, the surfaces will be cleaned using water. Neutralisers such as soda ash (sodium carbonate), which is alkaline, can be handy in counteracting the effects of sulphuric acid. Protective gear will also be worn during the clean-up process. The inadvertent touching of toxic chemicals will be handled by washing hands with plenty of clean water. An eyewash will be used to rinse the eyes thoroughly before seeking medical help.
If this procedure will involve ‘lone working’ has the risk assessment taken this into consideration?
There is a possibility of ‘lone working’ in the experiment, which has been considered in the risk assessment. However, ‘lone working’ will not be a significant problem because of the use of relatively dilute chemical reagents. In addition, automated micropipettes will be used to minimise the formation of aerosols and inhalation of noxious fumes. Adequate personal protective equipment will be provided. Therefore, there is no need for extra measures to assuage the risk.
Update:
The described risk assessment is recent based on the available information about the chemical substances. The procedures do not require major modifications.
Pipette Performance Check
What is the importance of this task?
A pipette performance check monitors the functioning of a pipette. This exercise is important because it ensures that pipettes are working as intended by manufacturers. Consequently, accurate fluid delivery is guaranteed with each use, which contributes to the accuracy and precision of results. Immunological assays are sensitive to variations in the volumes of reactants. For example, in competitive ELISA, the binding of an antigen to its antibody may be affected by the concentrations of the antigen and antibody. Therefore, it is necessary to ensure that correct volumes of reagents are used. Checking pipette performance facilitates easy troubleshooting if experiments do not work out as expected because the investigator is sure that correct volumes of reactants were used all through.
What is GLP?
GLP is an abbreviation for good laboratory practice. It comprises a series of guidelines designed to assure the quality and dependability of non-clinical laboratory inquiries. Such inquiries are necessary before attaining research or marketing authorisations for products that are regulated by government entities. Specific examples where GLP comes to play include drugs and non-pharmaceutical commodities such as food additives, levels of controlled substances in consumer products and medical gadgets. GLP does not include clinical inquiries. Furthermore, it is usually implemented as a quality management routine and not a scientific management system. Pertinent features of GLP comprise yardsticks for study behaviour, data collection and reporting of results.
What % error is acceptable when pipetting and is the acceptable % error similar for all pipettes?
When pipetting, the acceptable % error is 2% of the expected volume. This limit is recommended by the ISO 8655-2 standard, which is applied in pipette testing. However, other standards suggest that the acceptable error is 10%. It appears that the range of acceptable error differs with the size of pipette used. For example, a 100-1000 μL adjustable pipette has a systematic error of ±2.0 μL at the 100 μL setting, which is equal to 2% of the entire volume. However, assuming a similar error at the 1000 μL setting means that the error becomes 0.2%. Therefore, the error reduces when measuring larger volumes.
Defining % CV
CV is an abbreviation for coefficient of variation, which is obtained by getting the quotient of the standard deviation and the mean and multiplying by 100. CV facilitates comparisons between the level of deviation between groups of data despite having different means. When the %CV is high, it is an indication of big differences within a set of data. Conversely, low %CV values suggest that there is little variation. Furthermore, low %CV shows that the measurements are precise, which is crucial to the reproducibility of an experiment. A researcher should investigate the experimental process and identify sources of variation if high %CV values are recorded.
Define accuracy and precision?
Accuracy alludes to the closeness of a measured parameter to a known point of reference. Precision, in contrast, refers to how close a set of measurements are to each other. Precision occurs when the same measurement is obtained when an item is measured more than two times. A precise measurement may not always be accurate because and vice versa. Reproducibility of experimental findings is an aspect of precision that is often desirable. The results obtained in the pipette performance check were accurate because they did not differ significantly with the known pipette volumes. Additionally, the recorded errors were in the range of the standard error for pipettes. Nevertheless, there was no precision because varying values were recorded with repeated measurements.
Reporting of ELISA Results
Table showing the average response, standard deviation and % CV at each concentration
Table 1. Unprocessed data.
Table 2. Average response, standard deviation (SD) and % CV at various concentrations.
Table to generate a normalised calibration curve
A calibration curve of normalized response against concentration
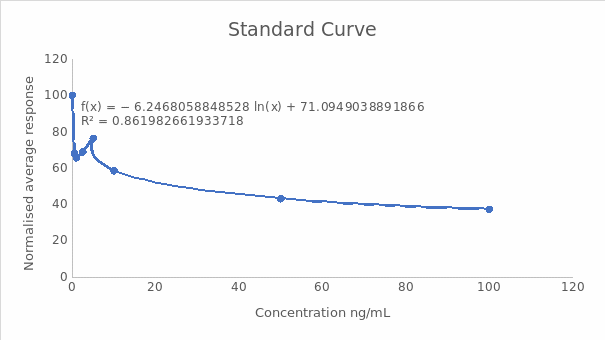
Midpoint (IC50) and the dynamic range (IC20-IC80)
- IC50 = ((Normalised response zero standard) – (normalised response highest standard) x0.5) + normalised response highest standard
- IC50 = (100-37.35) x 0.5+37.35
- ICS50 = 68.675, which was the same as 4.79 ng/mL
- IC20 = ((Normalised response zero standard) – (normalised response highest standard) x 0.8) + normalised response highest standard
- IC20 =100-37.35 x 0.8+37.35
- IC20= 87.47, which was equal to 0.34 ng/mL
- IC80 = ((Normalised response zero standard) – (normalised response highest standard) x0.2) + normalised response highest standard
- IC80 =100-37.35 x 0.2+37.35
- IC80= 49.88, which was 19.7 ng/mL
IC20 to IC80 corresponded to the dynamic range, which was between 0.34 ng/mL and 19.7 ng/mL.
The concentration of chloramphenicol from three unknown samples
Interpolation of the standard curve gave the concentrations of the unknown solutions as:
- Unknown 1= 9,358.12 ng/mL
- Unknown 2= 6802.19 ng/mL
- Unknown 3= 32.19 ng/mL
A comparison of results within the group between person 1 and person 2
Person 2 reported the following values for the unknown samples:
- Unknown 1= 1,498,539.22 ng/mL
- Unknown 2= 246,339.67 ng/mL
- Unknown 3= 21.7 ng/mL
There were similarities between the values obtained by person 1 and 2. It was noted that the concentrations of unknown samples 1 and 2 were extremely high. The normalised response values for these samples exceeded the range of the standards and could not be interpolated directly from the calibration curve. Instead, the equation of the line was used to compute the concentrations. This observation meant that unknown samples 1 and 2 were highly concentrated and required to be diluted before analysis. Therefore, the concentrations of these samples exceeded the limit of detection for the test.
Description of the microarray
The two sets of microarrays are designed for the measurement of chloramphenicol concentrations in samples. The first pair of dots in A represents fluorescence from labelled bovine serum albumin (BSA). These dots are the positive controls for the experiment and have also been placed strategically at the end of the microarray. The outlines of the buffer spots work as negative controls to facilitate the identification of non-specific binding, which would manifest as fluorescent signals outside the circles. However, if the positive control does not produce adequate fluorescence, then the test can be considered invalid. The control spots containing BSA are expected to generate a fluorescent signal that corresponds to a known positive sample. The second, third, fourth and fifth sets of dots are supposed to hold the samples under investigation. Therefore, fluorescent signals from these zones are attributed to the binding of the analyte to analyte-specific antibodies. The label shows that the four sets of dots contain 100ug/ml of chloramphenicol conjugate, which explains why the level of fluorescence in all the circles appears equal. This fluorescence resembles that generated by the positive controls, which confirms that the test is valid.
Similarly, the sets of two dots at the beginning and the end of the cartridge print area in microarray B show fluorescence from the positive controls (labelled BSA). Faint fluorescent signals can be seen in the test area containing the analyte. This fluorescence is less than that produced by the positive controls, which suggests that the samples do not contain chloramphenicol.
5 items to consider before implementing a new immunological method as a screening test for chloramphenicol
The most important item to consider is the cost of the assay. The overall costs of running the assay should not exceed the expected benefits. Useful factors that can contribute to the overall cost include the cost of procuring the test kits, chemical reagents and equipment. The cost of data analysis and interpretation and reporting should also be feasible.
The proposed immunological technique should be sensitive to chloramphenicol. High levels of sensitivity will avoid the production of false negatives. On the other hand, the test should not be too sensitive to non-specific binding of analytes because that would cause false positives.
It is important to consider the limits of detection of the test before adopting it. This aspect is important because it guarantees that the test outcomes meet established food and safety benchmarks. Recognised limits of detection of the test ease the identification of noncompliant food samples. For example, if the test has a narrow limit of detection, highly contaminated food samples may not be identified easily.
The overall productivity of the assay should also be considered. Sometimes there is a need to analyse many food samples in a short time. Therefore, it should be possible to process many samples using the new technique in the shortest time possible.
Ultimately, before introducing a new test method, the quality of the outcomes should be guaranteed. Consistent results should be obtained to promote the reproducibility of the assay. Large variations in the quality of data could have a negative impact on decisions that depend on the outcomes of the test. For instance, false positive outcomes could lead to the rejection of quality food products, thereby leading to unnecessary economic losses.