Introduction
The provision of palliative care to patients with terminal illnesses such as cancer has proved to be difficult to due excessive medicalization and the fear of death. According to Masman, Van Dijk, Tibboel, Baar, and Mathot (2015), morphine, haloperidol, and midazolam are common palliative drugs, which are administered at high doses, leading to significant adverse effects on patients. The solution to this problem is the incorporation of cannabinoid-based drugs because they are not only safe but also effective in the management of chronic diseases such as cancer (Hill, 2015). As a result, the purpose of this study is to compare the efficacy of morphine as a standard drug with marijuana as a novel drug in the management of pain in patients with cancer.
Research Question
In patients with cancer, what is the efficacy of marijuana on relieving pain compared to morphine when administered within two weeks? In this perspective, the study hypothesized that marijuana is more effective in relieving pain in patients with cancer when compared to the use of morphine.
Method Design Study Overview
Primary outcome
The primary outcome of the study is the degree of pain after the administration of morphine and marijuana. In measuring pain, the study will use the Brief Pain Inventory (BPI) because it considers multidimensional factors (Cluxton, 2019). BPI has four 10-point Likert items, which measure the highest and the least degrees of pain in the previous 24 hours, as well as the average and the current levels of pain (National Palliative Care Research Center, 2019). The degree of pain ranges from 1 to 10, representing the lowest and the highest levels, respectively.
Sample size calculation
The sample size of the study will be calculated based on the finite population of patients with cancer under palliative care in the hospital. Machin, Campbell, Tan, and Tan (2018) report that the margin of error, the size of the target population, confidence interval, and the proportion of the population are factors that determine the sample size. By using 70 as the target population of patients, 95% confidence interval, 5% margin of error, and 95% proportion, the sample size will be 36 participants.
Duration of the study
The duration of the study will take two weeks in which the level of pain among patients with cancer will be assessed before and after treatment. The period of two weeks is adequate to evaluate the changes in the degree of pain following the administration of morphine and marijuana.
Data collection procedure
In data collection, pre-treatment and post-treatment points will be used in the measurement of the degree of pain. Specifically, a fortnight’s interval will be used to differentiate the data collection points of pre-treatment and post-treatment. After sampling, participants will be randomized into two groups for morphine treatment (n = 18) and marijuana treatment (n =18). The pain will be measured before treatment and then drugs administered to patients as palliative care for two weeks after which the post-treatment pain levels will be measured.
Protection of participants
The study will seek ethical clearance from the ethics review board of the hospital to protects participants in the study. Protection of human subjects requires the use of written informed consent among competent participants with the allowance of voluntary participation and assurance of the confidentiality of data (Manti & Licari, 2018). In this view, the study will select adult patients with cancer who are competent to afford written informed consent to participate. Moreover, the study will allow these patients to participate voluntarily and protect their information from unauthorized access.
Target population
The participant population comprises patients with cancer who receive palliative care from the hospital. This population is appropriate to the study because patients with cancer have chronic pain, which requires the use of painkillers as an effective management intervention.
Selection criteria
As the selection criteria, the study will consider patients with cancer who experience severe levels of pain. To ensure the competency of these patients, the study will select adults with their ages ranging from 20 years to 60 years. Furthermore, these patients should be already enrolled in a palliative care program for more than six months for them to understand their levels of pain.
Sampling method
In sampling the participants, the study will use the purposive method because patients with cancer in the hospital have diverse complications. In the purposive sampling method, the study will select patients with chronic pain, competent adults (20-60 years), and those who have enrolled in a palliative program for more than six months.
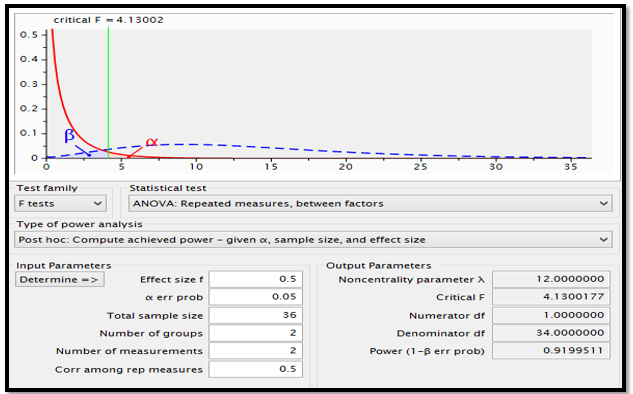
G*Power analysis
Power analysis using G*Power shows that with the sample size of 36 randomized between two treatments of morphine and marijuana with measurements before and after interventions and moderate size effect (0.5), the power of the study is 0.92, as shown below (Figure 1). According to Gupta, Attri, Singh, Kaur, and Kaur (2016), a power greater than 0.8 ensures that there is a significant difference between the treatments of various groups. In this case, a power of 0.92 implies that the difference in treatments of morphine and marijuana could be evident in 92% of the time.
Intervention
The study will involve the treatment of participants with marijuana (new treatment) and morphine (control treatment). Since these drugs are painkillers, the primary outcome of the study is the level of pain in response to varied treatments. The data will be collected by assessing the level of pain before the treatment and two weeks after the treatment.
Data analysis
Comparative analysis of the level of pain before and after treatment in two groups will be done using repeated-measures analysis of variance (RM-ANOVA). As outputs of RM-ANOVA, both descriptive statistics and inferential statistics would indicate if marijuana is comparable to the standard palliative treatment with morphine among patients with cancer.
Review of Design Choices
Choice of Problem
The use of medications in palliative care, such as morphine, haloperidol, and midazolam, has led to adverse effects owing to their high doses and addiction (Masman et al., 2015). The common analgesic used in the management of pain among patients with cancer is morphine (Tian et al., 2016). Since marijuana has analgesic properties, it is safe and effective in the treatment of pain (Hill, Palastro, Johnson, & Ditre, 2017; Nugent et al., 2017; Hill, 2015). So, the literature review shows the basis of the research question of the study.
Choice of Design Approach & Methodological Controls
Since the hypothesis is that marijuana is more effective in relieving pain in patients with cancer than morphine, the study used pre-post trial design. Fregni and Illigens (2018) assert that repeated-measures design is advantageous because it reduces variability, increases validity, and allows the use of small sample sizes.
Choice of Setting
The study setting is a hospital environment because patients with cancer attend and receive palliative care. Additionally, the hospital offers resources, technical expertise, and treatment interventions required by the study. The hospital eases the process of data collection since it is a convenient location for patients to present themselves for palliative care.
Choice of Study Duration
The study chose two weeks as an adequate duration for both morphine and marijuana to demonstrate their considerable efficacies in relieving pain. The analysis of the efficacy of cannabinoids shows that at least two weeks is essential for them to alleviate neuropathic pain among patients (Vuckovic, Srebro, Savic, Cedomir, & Milica, 2018). Also, two weeks is a short duration to protect patients from the inefficacy of marijuana when compared to the standard treatment.
Choice of Intervention
The selected intervention is the use of marijuana in the management of pain among patients with cancer. The intervention entails oral administration of 10 mg dose per day of morphine and 2.5 mg dose per day of cannabinoids for two weeks (Vuckovic et al., 2018; Tian et al., 2016).
Choice of Comparator
To protect participants from lack of treatment, the study chose standard treatment by using morphine as an active control. Masman et al. (2015) assert that morphine is a standard painkiller that is common in the management of pain.
Choice of Population
The study selected patients with cancer because they suffer from chronic pain, which requires palliative care for effective management (Cluxton, 2019; Tian et al., 2016). The use of different populations would not depict apparent differences in results due to low levels of pain.
Choice of Sampling Approach, Recruitment, and Power
The application of the purposing sampling method is relevant to the study because it enables the attainment of the calculated sample size and the selection of the right participants. The use of a different sampling method would reduce the external validity of outcomes. With the target population of 70 patients, the calculated sample size should be 36 participants who are randomized equally to treatment and control groups with significant power of 92%.
Choice of Assignment
As the study has the control and the treatment groups, the sample size will be allocated randomly to each one. The random allocation of participants ensures an equal chance of representation and eliminates biases associated with sampling procedures.
Choice of Evaluation Plan
In the evaluation of pain, a primary outcome, the study will use an established scale called Brief Pain Inventory, which is not only valid but also reliable (National Palliative Care Research Center, 2019). Furthermore, the scale is short because it has four Likert items, and the measurement procedure of pain is not only simple but also a non-invasive administration of a survey.
Choice of Analytic Strategy
To test the hypothesis that marijuana is more effective in relieving pain in patients with cancer than the use of morphine, the study will use repeated-measures ANOVA. This inferential test is relevant to the study because it compares the level of pain due to the effect of standard treatment (analytic control) and the novel treatment. Repeated-measures ANOVA requires a continuous variable to be measured more than once following an intervention to permit a pre-post analysis of outcomes (Fregni & Illigens, 2018)
Choice of Interpretive Strategy
Doctors, clinical officers, and statisticians will be involved in the interpretation of findings. Doctors and clinical officers would interpret the levels of pain measured and determine if they have any clinical significance. In contrast, statisticians will interpret if the treatments cause significant declines in the levels of pain among patients with cancer. In conclusion of the findings, the degree of pain will decline, and the statistical significance of the decrease will be considered.
References
Cluxton, C. (2019). The challenge of cancer pain assessment. The Ulster Medical Journal, 88(1), 43-46.
Fregni, F., & Illigens, B. M. W. (2018). Critical thinking in clinical research: Applied theory and practice using case studies. New York, N.Y: Oxford University Press.
Gupta, K. K., Attri, J. P., Singh, A., Kaur, H., & Kaur, G. (2016). Basic concepts for sample size calculation: Critical step for any clinical trials! Saudi Journal of Anesthesia, 10(3), 328-331. Web.
Hill, K. P. (2015). Medical marijuana for treatment of chronic pain and other medical and psychiatric problems, a clinical review. Journal of American Medical Association, 313(24), 2474-2483. Web.
Hill, K. P., Palastro, M. D., Johnson, B., & Ditre, J. W. (2017). Cannabis and Pain: A Clinical Review. Cannabis and Cannabinoid Research, 2(1), 96-104. Web.
Machin, D., Campbell, M. J., Tan, S. B., & Tan, S. H. (2018). Sample sizes tables for clinical, laboratory and epidemiology studies. Hoboken, NJ: John & Wiley.
Manti, S., & Licari, A. (2018). How to obtain informed consent for research. Breathe, 14(2), 145-152. Web.
Masman, A. D., Van Dijk, M., Tibboel, D., Baar, F. P., & Mathot, R. A. (2015). Medication use during end-of-life care in a palliative care center. International Journal of Clinical Pharmacy, 37(5), 767-775. Web.
National Palliative Care Research Center. (2019). Brief pain inventory (short form). Web.
Nugent, S. M., Morasco, B. J., O’Neil, M. E., Freeman, M., Low, A., Kondo, K., … Kansagara, D. (2017). The effects of cannabis among adults with chronic pain and an overview of general harms: A systematic review. Annals of Internal Medicine, 167(5), 319-331. Web.
Tian, C., Wang, J. Y., Wang, M. L., Jiang, B., Zhang, L. L., & Liu, F. (2016). Morphine versus methylprednisolone or aminophylline for relieving dyspnea in patients with advanced cancer in China: a retrospective study. SpringerPlus, 5(1), 1-6. Web.
Vuckovic, S., Srebro, D., Savic, V. K., Cedomir, V., & Milica, P. (2018). Cannabinoids and pain: New insights from old molecules. Frontiers in Pharmacology, 9(1), 1-19. Web.