Case report
This particular case talks about a male patient in his 40s. He was admitted to the emergency department of a hospital after a sudden onset of severe pleuritic chest pain. The patient was seen to be very nervous. His blood pressure was shown to be above normal range. He was also experiencing difficulties in breathing “dyspnoea”, chest problems, excessive perspiration and could only manage to speak incomplete sentences while at the emergency department and during the imaging exams. However, he was accompanied by his friend who helped him during the diagnosis.
His clinical history revealed that he was a non smoker; it was also revealed that he had several such painful bouts before. He again had ambulatory back pain which often increased when he sat in one position for a long time. The pain was however alleviated when the patient modified his position. Initially, Pleural fluid aspiration was done to relieve him of the painful bouts he was experiencing. During his examination the doctor had requested for plain chest x-rays views: PA, LATERAL AND LATERAL DECUBITUS. The cause for his pleural effusion was however not included on the request form.
Pleural effusion is defined as the accumulation of fluid in the pleural space. It is usually asymptomatic but pleuritic chest pain of varying degree can occur in patients. Pleural effusion is further amplified when more fluid enters the pleural space than is taken away (Richard 2006). The clinical manifestations of pleural effusion vary considerably and are often related to the underlying disease processes. The most commonly associated symptoms are progressive dyspnoea, cough (typically non-productive), and pleuritic chest pain. The pleuritic chest pain if related with pleural irritation is felt as a localized, sharp, and severe pain which is radiating in nature. This is exacerbated by deep irritating pain and coughing. The development of the effusion may however relieve the pleuritic pain as more fluid leads to less friction between the two membranes. On the other hand, patients with dyspnoea usually indicate a larger effusion.
In pleural effusion, chest pain directly relates with pleural irritation which aids in making diagnosis as effusions rich in transudates do not cause pleural irritation (Abrahamian 2008). The signifies the identifying and diagnosing ranges from incidental cardiopulmonary manifestations of underlying malignant pathologies (Murray 2004).
Clinical history
Even though various illnesses may bring about a pleural effusion, the main regular causes particularly in the US are cancer congestive cardiovascular ailments and pneumonia. The investigative workup of an ailing individual who has this problem depends entirely on the likely causes of the condition in that person. Like in our case study, the record/history and the bodily tests are essential in directing the evaluation and diagnosis of pleural effusion. Some factors linked to physical examination must again get special attention. In such situations chest examination characteristically exposes dullness to hitting, lack of fremitus, weakened breathing sounds and/or their nonexistence. In such a case the following symptoms might be evident: “distended neck veins, an S3 gallop, or peripheral edema suggesting congestive heart failure, and a right ventricular heave or thrombophlebitis suggesting pulmonary embolus. The presence of lymphadenopathy or hepatosplenomegaly suggests neoplastic disease, and ascites may suggest a hepatic cause” (Light, 2002).
Because situations save for pleural effusions might generate comparable radiologic results, optional imaging studies are often essential in confirming that a pleural effusion is there. “Ultrasonographic studies or lateral decubitus radiographs are most commonly used, but computed tomographic (CT) scans of the chest allow imaging of the underlying lung parenchyma or mediastinum can also be employed” (Roper 2005).
Signs of Thoracentesis
The sign of analytical thoracentesis shows the existence of a major medically significant pleural effusion. This is generally over “10mm thick on ultrasonography or lateral decubitus radiography with no known cause. If a patient is presented with congestive heart-failure and bilateral effusions of similar size, is febrile, and has no chest pain, a trial of diuresis can be undertaken” (Newman 2000). Because over 80% of people with pleural effusions brought about by congestive-heart-failure display signs of bilateral-pleural-effusions, thoracentesis is signified when the condition is unilateral. Roughly 76% of effusions caused by congestive heart failure are determined in 2 days following the onset of diuresis. When this condition continues for more than 72 hours, thoracentesis is signified.
Initially thoracentesis is done for the reason of identification; except if patients have difficulties of breathing while in one position (like in our case), this will necessitate therapeutic thoracentesis to get rid of about 1500 ml fluid signified. Thoracentesis can even be done at the patient’s bed with the help of analytical imaging. Ultrasonographic control is signified if intricacy is encountered in getting pleural fluid or when the effusion is minute. It is still indecisive whether the employment of ultrasonography reduces the occurrence of pneumothorax after thoracentesis. The degree of experience of the person operating is perhaps more vital than whether ultrasonography is applied. Again it is not compulsory to carry out chest radiography regularly after thoracentesis except if air is obtained through the thoracentesis; “coughing, chest pain or dyspnoea develops, or tactile fremitus is lost over the superior part of the aspirated hemithorax” (Newman 2000).
The gross manifestation of pleural fluid presents important information. A bloody emergence of pleural fluids constricts the differential analysis. As shown by Bluff, in a sequence of about twenty one cases of pleural effusion that show bloody fluid, “12 are due to cancer, 5 to pulmonary embolism, 2 to trauma, and 2 to pneumonia. Turbidity of the pleural fluid can be caused either by cells and debris or by a high lipid level. The odour of the pleural fluid also provides useful information” (Bluff, 2005). A fetid odour shows that the person probably had the infectivity as a result of anaerobic bacteria, and a urine smell shows possible urinothorax.
Pathology
Based on protein concentration, effusion can be divided into transudates (<25g/l) and exudates (>35g/l). Effusions rich in transudates are mainly due to the increase in venous pressure such as in cardiac failure and fluid overload (Richard 2006). Other causes are hypoproteinaemia (cirrhosis) and hypothyroidism. Effusions rich in exudates are mainly caused by increased amount of leakiness in the pleural capillaries secondary to infection (pneumonia, tuberculosis) or cancer like bronchogenic carcinoma (Saladin 2004). Exudative effusion is however more harmful than transudative effusion like the former requires more extensive evaluation and the treatment is aggressive.
Pleural effusion indicates an underlying pathology that may be primary or secondary due to another organ disease. This may be acute or chronic. The mechanisms that play a major role in formation of pleural effusion are: reduction of pressure in pleural space; restricted expansion of the lungs; decreased intravascular oncotic pressure; increased capillary permeability; infection; altered permeability of pleural membranes; inflammation; trauma; gradual but consistent increase in pleural fluid oncotic pressure from an existing pleural effusion leading to further increment in fluid accumulation; lymphatic blockage; increased capillary hydrostatic pressure and iatrogenic pathology. Normally 1ml of pleural fluid is present between the two layers establishing a balance between two physiological process namely hydrostatic and oncotic pressure and a sound lymphatic drainage.
Pleural fluid is continuously produced by the parietal pleura which are absorbed by the visceral pleura and by the lymphatic parietal pleura. Three physiological processes based on pressure system affect the circulation of the fluid which is: hydrostatic, colloid osmotic and tissue pressure. Alteration of 1 or more of these factors causes abnormal accumulation of fluid in the pleural space and is the primary mechanism of transudative effusions (Lababede 2007). Other modes of development of effusion are due to injury to the pleura or sub pleural lung parenchyma which in turn can cause increased vascular permeability. This mechanism holds in exudative effusion production scenario, such as effusion associated with pneumonia and infarction (Rubins 2008).
Considering imaging the patient
Plain chest x-rays, Computed Tomography and ultrasound are what the radiographer needs in making a diagnosis and they also serve as therapeutic options.
General Radiography/X-rays
Normally pleural effusion appears as a homogenous density or shadow and lies outside the lung which is bounded by a sharp concave interface. Free or mobile pleural effusion tends to collect in the most dependent portion of pleural cavities, hence varying with the position of the patient. The smooth edge between lung and pleural fluid can be recognized well on an image that has been adequately penetrated by x-rays and provided that the lung is well aerated. Fluid in the pleural cavity assumes similar appearance and does not depend on whether the fluid is transudate or exudate. Small effusions blunt the costophrenic angles whereas large effusions are seen as water dense shadows with concave upper borders, but this feature may be horizontal instead if there is an associated pneumothorax.
Pleural effusions that can freely move has a different appearance depending on the position i.e. whether supine position or upright (PA). When the patient is supine, posterior layered fluid causes an ill defined increase in the density of hemi thorax and the outline of hemi diaphragm is disturbed. Occasionally standard Lateral and PA may not show small effusions such as when the fluid is less than 300 ml. In this case, lateral decubitus view plays a major role and provides the necessary information radio-logically. The mechanism acts in the sense that, when in the lateral decubitus position, the free fluid settles along the dependent lateral chest wall. Large effusions also tend to displace the trachea and mediastinum to the opposite side which may be related to lung collapse (Murray 2004).
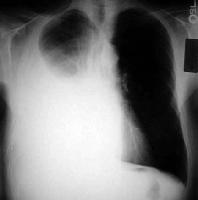
Computed Tomography
The basic principle in CT remains the same i.e. fluid appears the same without any relation to whether it is a transudate or exudate. The density of pleural fluid is homogenous and appears lower density than most tissues. However, if CT cannot be done then a lateral decubitus can be an alternative as it indeed provides a picture of the effusion (Richard 2006). Usually this happens when the patient is in supine position for this exam. If the underlying pathology is cardiac failure, small bilateral pleural effusion is frequently seen. But if congestive heart failure is the cause of effusion then it is present bilaterally (Abrahamian 2008).
Helical CT
Helical CT scan also identifies alternative explanations for the development of pleural effusions (Richard 2006).
Ultrasound
This modality recognizes pleural effusion as a transonic area between the lung and chest walls. It could be done with the patient in any position. Ultrasound identifies effusion as numerous echoes within the fluid if the fluid is due to pus. It is usually utilized in diagnostic and therapeutic applications such as US guided aspiration (Murray 2004).
Treatment
The pathology is managed by treating the underlying cause. Drainage of the fluid is a preferred method of choice and can be done repeatedly as per the specific requirements. The drainage should be done slowly during US guided aspiration with a gauge needle attached to a syringe or a chest tube which can also be used for the same purpose. Thoracentesis needle can also be used to drain the effusion if it is in large volumes. Pleurodesis with talc and tetracycline (antibiotic) is advisable for recurrent effusions.
The position of the patient is significant when carrying out drainage of the pleural effusion (Murray 2004). Thoracentesis should be performed in all patients with more than a minimal pleural effusion (i.e., larger than 1 cm height on lateral decubitus radiograph, ultrasound, or CT) of unknown origin (Richard 2006) except if patients are known to have obvious heart failure. Transudative effusions are managed conservatively by judicious use of drugs. Drugs should be carefully administered as medications since they also tend to cause effusions (Lababede 2007).
Patient preparation
Patient preparation before CT scanning is quite simple. The patient is not supposed to take or drink anything for about 6 hours preceding the scan test. Any metallic objects in the body should again be removed since they can hinder with the image taking procedure. For patients diagnosed with claustrophobia, calming prescription is advisable before scanning is done.
Scanning protocol of CTA
Normally CT scanning is used for examining pleural effusion on the chest. For this test, an X-ray specialist puts the patient on a slim, level table. The patient is placed in a way where he/she exposes the chest for better image taking. The table then will begin to go in and out of the X-ray machine, while chest pictures are taken by use of X-ray beams. This is done at different angles.
CT scanning can verify the presence of pleural effusion. “If the test images show swellings in the membranes around the lungs or a fluid accumulation in this area, pleural effusion can be confirmed. Once this condition has been diagnosed, the correct treatment option will be chosen” (Kohs, 2000).
Contrast protocol for the examination
Many imaging protocols have been identified and used in diagnosing. The protocols illuminate the analytical requirement of CT contrast standard or the blending of contrast-improved lessening rectification CT scans and PET. Additionally, breathing protocols have been devised in such a way that they prevent the moving of artefacts, particularly up in the abdominal organs. Up to this time, CT termination protocols are favoured even though emission scan is carried out in shallow-free-breathing and there might be a diminished analytical CT quality, particularly in the lung tissues.
A question arises here whether it is achievable to do a whole analytical CT examination scan with dissimilar contrast stages in amalgamation with the whole body PET discharge scan. Getting a devoted breathing-protocol ought to allow a “diagnostically powerful co-registration of PET and multiphase CT scans. Such a method would require an ultrafast multi-slice CT scanner that is equipped with a 4-row or 16-row multi-detector system and that can scan without increased examination time” (Kohs 2000).
Strategies took to reduce radiation
Every CT scanner includes an X-ray tube which produces an X-ray beam during the scanning process. Radiation contact to an individual being scanned is decided by the features of the X-ray beam, which in turn relies on the constraints being employed for the CT scanning. Even though lessening scanning parameters like the X-ray tube existing and scan period lessens radiation contact, they again affect the analytical quality of pictures generated. This is especially true if the scan parameters are not attuned cautiously. Therefore, while minor radiation dose CT pictures could give analytical results, sometimes they might not be esthetically pleasing because of the average radiation dose. Doctors and radiologists must recognize that this scans intend to achieve indicative superior images with the least likely radiation contact. They should not concentrate on attractive images at bigger radiation exposure than is needed. This task is hard since there is an obvious lack of instructions on standard scan performance to be employed for getting a routine CT scan.
To reduce radiation the following measures should be put in place; CT tests must be narrowed to cautious indications having eliminated inappropriate request of CT scans, “protection of radiosensitive organs like breasts, eye lenses, thyroid and gonads is especially relevant in pediatric patients and young adults, as these parts frequently lie in the pathways of X-ray beam” (Muller, 2002), and doctors with radiologists should emphasize CTs that reduce radiation exposure and adjusted as per the individual’s age and size. As shown by Muller:
Radiation dose associated with HRCT of chest is much higher than a routine chest scan. Even with reduced radiation dose scanning technique, the radiation dose of HRCT can exceed the radiation dose of chest radiography by 100 times. Therefore, HRCT should be restricted to carefully selected indications such as investigation of suspected interstitial lung disease, airspace diseases and in immune-compromised patients with acute parenchymal abnormalities, where differential diagnosis or a specific diagnosis can be made (Muller, 2002).
Image display, appearance and analysis (2D/3D reconstruction)
In pleural effusion digital-geometry-processing is employed to produce a 3D picture of the patient’s chest from the inside. This is done from a huge series of 2D displays taken on one axis of rotation.
Computed tomography gives an amount of data that can be controlled, via a windowing process to exhibit different physical formations supported on the aptitude to obstruct the X-ray beam. Even though in the past the images produced were on a slanting plane, orthogonal to the extended axis of a body, current scanners permit this quantity of data to be re-arranged in a variety of planes or even as quantitative three dimensional illustration of construction that helps to ease diagnosis.
Patient’s aftercare
In pleural effusion patient after care is essential for effective healing. It is important to evaluate a patient’s respiratory alteration and the pain in the chest especially during inspiration. It is also important to auscultate the patient’s lungs for reduced breathing sounds or pleural rub. The patient is also supposed to undergo chest percussion so that evaluation for the changes in tactile fremitus can be monitored. Monitoring of the pulse oximetry should also be included in the aftercare program.
In the aftercare program the patient is supposed to be put in point which maximizes aeration, normally an elevated Fowler’s position. The patient should be taught deep breathing and effectual coughing methods. Another thing involved in patient aftercare is how to splint the patient’s chest while coughing or how to carry out inducement spirometry.
If a patient needs thoracentesis or placing of a chest-tube, an explanation should be done on the procedure that follows. Emotional sustenance and the administering of analgesics should be done as required while carrying out positioning. Aftercare also involves the receiving of specimen tubes while the doctor gets rid of fluids.
Patients with chest-tube insertion should be monitored as per the kind of fluid drained. “Maintenance of fluid levels in suction control chambers and watching for bubbling in the water-seal-chamber must be monitored. If the bubbling stops evaluation whether the lung has completely re-expanded or if the drainage tube has become occluded should be done” (Dedierech, 2009).
An absolute respiratory evaluation after every procedure should be done on the patient as required. Amplified dyspnea, reduced or no breath sounds and painful stimulation might point out more effusion or the expansion of the pneumothorax.
Treatment and prognosis
More often than not, when treating pleural effusion, the major factor that is considered is the causative agent. For example, pleural effusion caused by heart failure is tackled by giving the patients some diuretics; pleural effusion caused by infections is treated by serving the patient with antibiotics and pleural effusion caused by use of a certain drug is handled by removal of the drug causing this condition. However, there are two conditions of pleural effusion that often require more special attention. These are Emphysema and Malignant effusion.
Emphysema comes about when infections in the pleural cavity results to severe inflammation, this leads to formation of adhesions in the pleural cavity. It is caused by bacterial pneumonia and it is always not infectious (also referred to as Para pneumonic effusion). However there is inflammation when the fluid is infected. This infection is eradicated by draining the fluid in the cavity. Therapy is then directed at always draining the fluid that often rapidly re-accumulates when thoracentesis is performed. The draining is done using a chest tube to keep the fluid off the cavity till the infection has cleared. This is the most common method of treating Emphysema; it is safer because complications are rare, only that chest tube placement is usually uncomfortable to the patients. Until the tube is removed patients are also administered with systemic analgesics till the time when the tube will be removed. In this case of pleural effusion, it is often not possible to exhaust all the fluid from the cavity, this is due to the formation of Fibrin Septa, which forms loculated effusion, and makes it impossible to reach the drainage site. This is dealt with through chemical means by administering chemicals that break up the fibrin adhesions such as streptokinase, or through a surgical removal (thoracoscopy) in which a surgeon opens up the pleural space and drains the fluid.
Malignant pleural effusions are often as a result of exposure to Asbestos elements that may have transformed from somewhere in the body e.g. the lungs. Treatment of malignant effusion mainly involves relieving the patient of chest pains and breathing difficulty. There are several ways to treat this condition and the choice with which to handle it depends on the specific case. Chemotherapy is used and if the disease responds, then the case may be solved. Thoracentesis is a process in which the fluid is suctioned off the cavity when it accumulates over and over again. If this fluid re-accumulates rapidly after it has been drawn out, Pleurodesis is done. This is an application of a sclerosing agent that makes the surfaces of the parietal and visceral pleura to stick together, limiting the room for the accumulation of the fluid.
Conclusion and recommendation
Pleural effusion is not a disease by itself and is more of a symptom or pathology due to a primary disorder afflicting a systemic organ. However its production and presence in the body suggest cardio-logical, pulmonary and malignant etiologies which should be investigated. Imaging modalities find their importance here as they aid in visualizing and diagnosing the indirect symptom as well as the primary disorder.
Pleural effusion is again linked to many systemic illnesses. “Thoracentesis to determine if the pleural fluid is a transudate or an exudate coupled with other appropriate diagnostic studies provides a diagnosis most of the time. Because pleural fluid findings are often nonspecific (except for positive cytology and bacteriology)” (Heffner 2007), the medical connection and reaction to therapy are decisive. It is not a must that pleural fluid studies should be ordered on all pleural effusion. Medical decision continues to be the key on this problem.
A thoracentesis ought to be executed in patients with this problem especially if it is of an unknown cause. However, there is an exception when the effusion is minute i.e. below ten mm on ultrasonography or the ailing person has congestive-heart-failure together with bilateral-pleural-effusions. As shown by Kinasewitz:
Ultrasonographic guidance for the thoracentesis is indicated if the effusion is small or if difficulty is encountered in obtaining fluid. If it is likely that the patient has a transudative pleural effusion, the only laboratory tests indicated are measurements of the lactate dehydrogenase and protein levels in the pleural fluid. If a patient has an exudative effusion, as indicated by a ratio of the pleural-fluid protein level to the serum level of more than 0.5 should be stained. Again a ratio of the pleural-fluid lactate dehydrogenise level to the serum lactate dehydrogenase level of more than 0.6, or a pleural-fluid lactate dehydrogenise level that is more than two thirds the upper limit of the normal for the serum lactate dehydrogenase level should be stained. The pleural fluid should be stained with Gram’s stain and cultured for bacteria (Kinasewitz, 2002).
Furthermore, these examinations are supposed to be carried out on exudative-pleural-fluid: entire and discrepancy cell counts, the capacity glucose, the evaluation for pleural-fluid-marker for tuberculosis especially when the effusion is primarily lymphocytic together with cytological examination.
If no analysis is apparent following the first assessment, the likelihood of pulmonary-embolus must be assessed; this particular analysis ought to be followed before a suggestive medical appearance of this condition. However if the analysis is still uncertain, consideration must be specified to making more persistent tests, the likes of thoracoscopy, “needle biopsy of the pleura, or open pleural biopsy” (Kinasewitz, 2002).
In this case-study, congestive heart failure could be possible, because the patient showed an indication of this condition in his medical records. Nevertheless, because the effusions are uneven and the pain is there, thoracentesis is signified. Exudative-effusion signs of cytological difficulty are often shown if cancer is a concern in cases where the patient has records of smoking; this also acts with age but this is not evident in our case. On the other hand if this evaluation is non-indicative, thoracoscopy or a different invasive analysis must be considered.
References
Abrahamian, F. M. 2008. Pleural Effusion. Web.
Bluff, Stanley. 2005. Diagnosis of Pleural effusion. New York, NY: Oxford university press.
Dedierech, Lazen. 2009. Pleural Effusion: Patient after care. Oxford: Pergamon Press.
Heffner, Jennings. 2007. Pleural effusion Diagnosis. Clin Chest Med, 70, no 1, (2000): 5-13.
Lababede, O. 2007. Effusion, Pleural. Web.
Kinasewitz, Gary. 2002. Pleural effusion Diagnosis. Journal of Pleural effusion, 5, no 2, (2002): 328- 341.
Kohs, Gregory. 2000. CT Scanning. Clinical CT practice, 2, no 14, 2000: 35-52.
Light, Richard. 2002. Disorders of the Pleura. Principles of internal medicine, 42, no 5, (2002): 54-96.
Muller, Jokoh. 2002. Radiation exposure in CT scanning. Medical sci, 19, no 9, (2002): 142-151.
Murray Longmore, I. B. W., Supraj Rajagopalan. 2004. Oxford handbook of clinical medicine. 6th ed. New York: Oxford.
Newman, Daniel. 2000. Thoracentesis in pleural effusion. Journal of Pleural effusion, 8, no 6, (2000): 136- 151.
Pleural Space. 2006. Web.
Richard W light, J. M. P. 2006. Diagnostic approach to pleural effusion in adults American family physician 73 (7): 1211. Proquest.
Roper, Willis. 2005. Diagnostic tests of pleural effusion. Clin Chest Med, 14, no 22, (2005): 142-186.
Rubins, J. 2008. Pleural effusion: treatment and medication. Web.
Saladin, K.S. 2004. Anatomy and Physiology: The Unity of Form and Function. New York. McGraw-Hill.