Abstract
There is a dire need to produce targeted drug delivery vehicles for efficient treatment especially in the treatment of carcinogenic cells. The current proposal envisaged investigating the pathways of cellular internalization of shape and size-specific particles in different cancer cell types, employing modern optical imaging techniques. “A top-down particle fabrication technique was employed to fabricate monodisperse submicron cylindrical particles. The internalization of cell incorporated PRINT particles was monitored via several bioimaging techniques, like confocal microscopy, flow cytometry, and transmission electron microscopy.” (Moriyama1 et al 2008) Through the series of experiments, it was revealed that the internalization of the drug delivery vehicles in the cancerous cells was due to clathrin-mediated endocytosis and macropinocytosis with some particles exhibiting a caveolae-mediated endocytotic pathway. It was further depicted that the surface charge of the particles played a major role in incorporation, and had a dominant effect on the rate of internalization, in most of the cells. The experiments revealed that there was a significant lack of cytotoxicity due to the incorporation of the particles used as vehicles for drug administration due to the manipulation of their shapes. Positively charged particles were found to have greater efficiency in terms of being internalized, and this was further authenticated by employing zeta potential measurements. It was also revealed that the positively charged particles were incorporated by endocytosis in the HeLa cells by means of several pathways, including clathrin-mediated and macropinocytosis, thus successfully employing optical imaging techniques with photolithography to produce targeted drug delivery mechanisms.
Introduction
The recent advances in nanotechnology are projected towards the phenomenon, that as we move towards smaller scales, the underlying laws of physics change and recent advances in this emerging technology has enabled us to measure and manipulate individual structures on the nanoscale employing scanning probe techniques, optical tweezers, and high-resolution electron microscopes (Dilwart, 2008).
Recently, there has been an increasing trend towards nanoscience and nanotechnology in order to address important issues of optics on the nanometer scale. The studies by Moriyam (2008) suggest that since the limit of diffraction poses as a handicap in focusing light to smaller dimensions, i.e. less than half a wavelength, it was thus not possible to interact optical imaging with nanoscale features. Research conducted by many scientists is targeted to ‘shrink’ the diffraction limit (confocal microscopy) or to even overcome it (near-field microscopy). She also suggested that in addition to the extrinsic dimensions of the particles, the intrinsic properties were also prone to be changed, and the side effects are depicted to become more prominent as one approaches nanoscales. Optical detection systems in nano-scale biomedical research studies have been exploited to study the naturally occurring differences in normal and cancerous tissues, in terms of their optical properties. However, if targeted, optically active agents are employed, they would further enhance the applicability and efficiency of optical biopsy procedures. William (2008), in his paper, presents the fact that light is employed for imaging, and recording measurements, and thus the time of measurement is decreased due to the fluctuations that are attributable to the living body. He proposes that the presence or absence of signals to be measured can be determined by a display of estimation signal and measurement signal simultaneously. The recent exploration and utilization of particles, such as nanocarriers for the delivery of therapeutics in vivo have led to tremendous improvements in the efficiency of different cancer and drug therapeutic approaches.
Jennifer and her coworkers (2008) have conducted several research studies pertaining to the delivery of drugs, and have conducted similar studies directed towards the ‘manufacture’ of nanoparticles to be employed as drug delivery vehicles. The different vehicles of drugs have created new options in the development of site-specific targeted drug delivery. DeSimone (2007) and his research group at the North Carolina University have been deeply involved in the synthesis of nanoparticles for drug delivery, and in studies pertaining to monitoring their release mechanisms. He has demonstrated a unique approach to fabricate monodisperse nanoparticles, thus modifying the traditional lithographic methods, which employ wet chemistry. Future studies should be directed towards control over size, chemical composition, uniformity, and targeted drug delivery. He has mentioned that additional challenges faced by scientists are that the drugs are delivered through unstable encapsulation (as they are not stable objects) and there is little control over size and shape, and release over time cannot be monitored. He has added a new dimension in lithographic studies employing bioimaging super-resolution technology, using nonwetting silicon templates. Rolland (2004) has emphasized that the pathways of internalization need to be taken under consideration, as it has been demonstrated in his research study that “clathrin-mediated, caveolae-mediated, macropinocytosis play a major role in the intracellular trafficking of drugs”.
“A key advantage of fluorescence microscopy is its ability to map the relative distribution of two or more of these constituents by labeling them with spectrally distinct labels” as proposed by Ellis (Eullis, 2006). She also mentioned that most of the cellular interactions take place in regions that are localized, and are too small to be probed using conventional optics, due to its limited resolution of 200 nm. Thus, new developments include the utilization of super-resolution multicolor fluorescent methods.
This study utilizes polymer and organic chemistry, biochemistry, and cell biology to investigate these mechanisms using a novel particle fabrication method called ‘PRINT’ (Particle Replication In Non-wetting Templates), coupled with the biomedical imaging techniques, Scanning electron microscopy, and florescent probing to detect and trace the drug, and monitor its release over time. This technique is promising, as due to the low surface energy of the Fluorocur that is used, we will be able to produce discrete, shape, and size-specific monodisperse particles that can be easily employed for targeted efficient drug delivery approaches. We will also explore the internalization mechanisms of non-targeted cylindrically produced particles in human cancerous cells (HeLa cells), generated by tissue cultured cell lines, followed by employing bioimaging techniques for monitoring drug incorporation and release.
Materials and Method
Preparation of PRINT Particles 20 ml of Fluorocur resin containing 0.1% of 2,2- diethoxyacetophenone was pooled in the center of an 8″ patterned master (silicon wafer) –(with features of 200 nm, or as required). This wafer can be purchased, or custom-made from Benchmark Technologies. This system was set up inside an enclosed UV chamber and purged with nitrogen—for 3 minutes. It was then exposed to UV- λ=365nm; >20mW/cm2 – to cure the wafer- for chemical cross-linkage formation, by means of UV energy. “The PFPE mold was removed from the master template by gently peeling it away from the silicon surface. 78% (w/w) PEG triacrylate + 20% (w/w) PEG monomethyl ether monomethacrylate + 1% (w/w) 2,2- diethoxyacetophenone + 1 (w/w) para-hydroxy styrene (PHS)–mix together-label as (a). A separate vial, filter 2-propanol through a 0.22 µm PTFE filter,- this is (b). 10% (w/v) of (a) was mixed with (b)—and 1 ml of this solution was sprayed onto a Fluorocur patterned mold – prepared above, — using an airbrush. A poly(ethylene) sheet was then placed over the 8″ mold—ensuring that the entire active area was covered.” (PubMed)—a function of this process: application of ‘pressure’, so that the solution is ‘pressed into’ the mold—lamination. “For harvesting the particles, Acetone was first filtered through a 0.22 µm PTFE filter. A drop of this filtered acetone was gently moved along the surface of the mold using a glass slide. The movement of the glass slide facilitated the release of the particles from the mold. The suspended particles were collected in a 50 ml Falcon tube and diluted to 50 ml with filtered acetone. The suspension was vortexed for 10 min and then centrifuged at 3200 rpm for 30 min. The supernatant was removed and particle pellets redispersed in 50 ml of fresh acetone by vortexing for 10 min followed by centrifugation for an additional 30 min. This process was repeated once more and after aspiration, the particles were redispersed in 5 ml of distilled water by sonicating the dispersion for 15 min, and the particle pellets were redispersed in 50 ml of fresh acetone to make up a final concentration of 10mg/ml.” (PubMed)
An analysis of the PRINT particles was conducting via zeta Potential measurements, TEM, and confocal laser scanning microscopy.
- Zeta Potential Measurements “The surface charge of PRINT particles was determined using a ZetaPlus Zeta Potential Analyzer (Brookhaven Instruments Corporation).
- Cell Lines and Maintenance HeLa cells were maintained in tissue culture MS media supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, and non-essential amino acids.” (PubMed)
- Confocal Laser Scanning Microscopy HeLa cells (50,000) were seeded in a T-25 flask for 24 h (37°C, 5% CO2). Following this, cells were washed three times with PBS. Cells were then incubated for 4 h (37°C, 5% CO2) with fluorescein-labeled PRINT particles. An Olympus FV500 confocal laser scanning microscope (Olympus Co Ltd) was employed to visualize the cells. Transmission Electron Microscopy (TEM) Internalization of particles was demonstrated using TEM. Cells were treated with PRINT particles and fixed in 2% paraformaldehyde/2.5% glutaraldehyde/0.15 M sodium. A diamond knife was used to section the monolayers parallel and perpendicular to the substrate. An LEO EM 910 transmission electron microscope operating at 80 kV was used to visualize the samples. Digital images were obtained using a Gatan Orius SC1000 CCD Digital Camera and Digital Micrograph 3.11.0 (Gatan, Inc.).
- Disruption of Clathrin-mediated Internalization Cells was incubated in serum-free MEM containing 75 μM Dynasore for 60 min at 37°C/5% CO2 and was then incubated in serum-free MEM/75 μM Dynasore containing 15 μg/ml particles for 60 min followed by analyses via flow cytometry.
Results
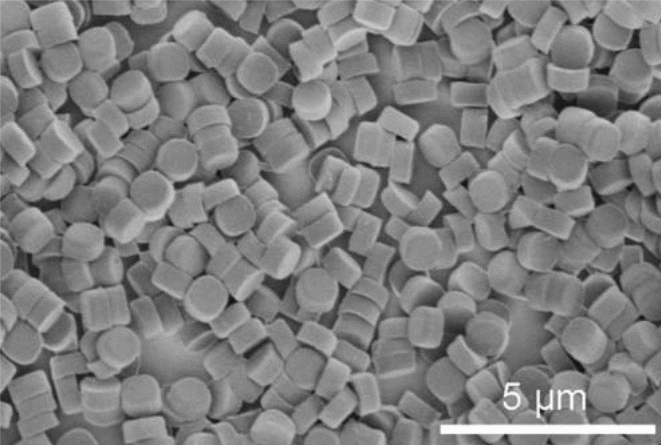
Discrete, monodisperse PRINT particles were obtained employing the nonwetting technology proposed by De Simone and his co-workers. As it can be clearly seen in Figure 2, uniform-sized particles were obtained, making this an efficient drug encapsulating technology. Figure 2 demonstrates a diagrammatic flow of the process used to ‘manufacture’ the particles.
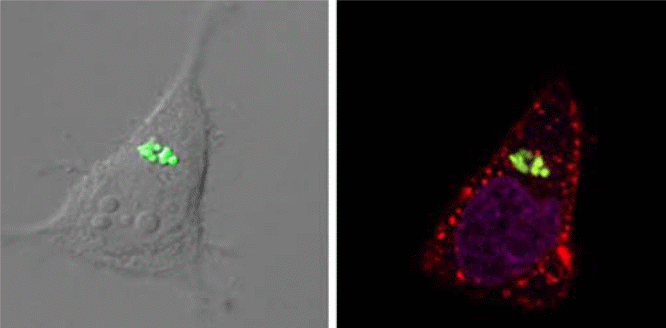
Transmission electron microscopy was employed to determine the average size and morphology of the nanoparticles, and they were depicted to have an average width of 1.00 ± 0.06 μm, and an average height of 0.68 ± 0.05 μm, with a meniscus on one side of the particle, that was due to the molding of the floor on the silicon wafer. The zeta potential of the positively charged particles was found to be +23 ± 3 mV using a Zeta Plus Zeta Potential Analyzer. Particle Internalization Internalized PRINT particles housing the PHS drug were observed using multiple techniques, including confocal microscopy, flow cytometry, and transmission electron microscopy (TEM). Figure 1 shows confocal micrographs of HeLa cells with internalized PRINT particles possessing a positive surface charge, and 1 μm PRINT internalized particles in HeLa cells using TEM.
Particle Internalization Pathways “Chemical inhibitors, were employed in order to study the pathways used by HeLa cells for internalization of the 1 μm positively charged PRINT particles.” (PubMed) Filipin was used for the investigation of particle internalization by a caveolae-mediated mechanism. “It was observed that when Filipin was used for inhibition of endocytosis, approximately, a 2% decrease in internalization was observed.” (PubMed) However, when Dynasore was used for probing, a significantly higher decrease in efficiency was observed, and a 35% decrease was seen, although Dynasore is a well-known inhibitor used for probing internalization via clathrin-coated pits. “The results obtained clearly suggest that multiple pathways are employed for the internalization of PRINT particles into HeLa cells, with the predominant pathways being clathrin-mediated endocytosis, and macropinocytosis.” (PubMed)
Dako flow cytometric measurements were then made in order to determine the percentage of cells residing in the PRINT particles, after a series of thorough steps of rinsing to remove any membrane-bound or non-internalized particles. “It was revealed that with the exception of the macrophage cell line where there was no preferential internalization observed, based on the charge of the particles.” (PubMed) It was also determined that at a particle concentration of 100 μg/ml, positively charged PRINT particles were found to be internalized into approximately all of the HeLa cells that were being analyzed; and on the contrary, it was observed that approximately 60% of the negatively charged particles were internalized at similar experimental concentrations. As can be seen in Figure 1, particles have been internalized, and the final results obtained depicted that macrophages did not play a significant role in the internalization of the PRINT particles, and also did not interfere in the incorporation of the drugs, and it was depicted that no significant amount of cytotoxicity was observed in the respective assays by the incorporation of PRINT particles by the HeLa cells.

Discussion
Current drug delivery Once an understanding of the particle’s fate in vitro and in vivo is known, major strides towards improving the efficiency of particle delivery technology can be made. However, due to lack of control over size, shape, and surface chemistry, manipulation of drug delivery mechanisms of drug delivery and release in the cells are hard to be determined. The optical imaging technology employed in this study has allowed us to fabricate particles with complete control over most of the parameters. We have been able to synthesize custom-made particles, that meet our requirements of size and shape and can be used as drug delivery vehicles. In the current study, we had synthesized nanoparticles via soft lithography approaches, in order to facilitate capturing images of the internalized PRINT particles using different microscopic techniques. Accurate monitoring of the incorporation of the drug in human carcinogenic cells was made possible by employing bioimaging techniques. “Likewise, the internalization of particles was further monitored using confocal microscopy, Scanning electron microscopy (SEM), and flow cytometry. Figure 1 depicts internalized 1 μm positively charged PRINT particles into HeLa cells by confocal microscopy.” (PubMed) In order to aid in the differentiation of membrane-bound particles from those that were internalized, different staining procedures were adopted. “3-D reconstruction of the particles was done to further demonstrate the internalized particles and their intracellular locations.” (PubMed)
SEM micrographs obtained in this study clearly show internalized 1 μm positively charged PRINT particles into the HeLa cells, and translocation of these particles in the HeLa cells. Similarly, both confocal microscopy and TEM exhibit the translocation of the positively charged PRINT nanoparticles to the perinuclear regions of the HeLa cells, and after a 60 minute incubation period, the majority of the internalized particles are observed to be localized in the perinuclear region of the cells. It was also observed that it is possible for more than one particle to be internalized into a single cell, as depicted by our results obtained by both confocal microscopy and SEM. In order to further understand the pathway of particles that have been once internalized, a series of inhibitors were used in the study to examine the endocytic pathways adopted by positively charge PRINT particles (Fig. 2). Based on the results obtained in this study, it can be concluded that most of the particle internalization in cells occurs due to an energy-dependent process as it has been observed 70% decrease in internalization occurs once NaN3/DOG is applied to the cells Previous studies (De Simone, 2007), suggest that NaN3/DOG is shown to block the synthesis of ATP synthesis impairs energy-dependent translocation in cells. “It was proposed that complete inhibition cannot be accomplished due to the presence of exogenous ATP and glucose. In this study, Dynasore was employed for the inhibition of clathrin-coated pit pathways by acting as a Dynamin GTPase inhibitor, and a decrease in particle internalization of approximately 35% was observed with this inhibitory process.” (PubMed) Moreover, when the macropinocytosis pathway was inhibited, an approximate 16% decrease in the particle internalization was revealed when during experimentation. “On the contrary, we had observed a 2%, decrease in internalization when a caveolae inhibitor, filipin was used.” (PubMed) The results obtained suggest that a targeted and controlled particle therapy could be successfully achieved.
Thus, particle internalization, coupled with imaging techniques employing nano-imaging techniques have been successfully employed in manufacturing, delivering, and monitoring the release of drugs, which have been seen to be successfully incorporated in carcinogenic cells, thus demonstrating the synergy of different techniques and principles in medical research.
References
- E.H. Moriyama1, G. Zheng and B.C. Wilson; Optical Molecular Imaging: From Single Cell to Patient Clinical pharmacology and Therapeutics (2008) Vol 84: No 2, 267-273.
- DeSimone. Nanofabricated particles for engineered drug therapies: A preliminary biodistribution study of PRINT nanoparticles; Journal of Controlled Release (2007) 121 10–18.
- Dilworth Y. Parkinson, Gerry McDermott, Laurence D. Etkin Quantitative 3-D imaging of eukaryotic cells using soft X-ray tomography Journal of Structural Biology (2008) 162 380–386
- Hideki Tamura, David C. Ng, Takashi Tokuda et al. One-chip sensing device (biomedical photonic LSI) enabled to assess hippocampal steep and gradual up-regulated proteolytic activities (2008). Journal of Neuroscience Methods 173 , 114–120
- Jennifer Y. Kelly and Joseph M. DeSimone; Shape-Specific, Monodisperse Nano-Molding of Protein Particles; Journal of American Chemical Society (2008) 130, (5438-5439.
- J.P. Rolland, E.C. Hagberg, G.M. Denison, et al, High-resolution soft lithography: enabling materials for nanotechnologies, Chem., Int. Ed. Engl. (2004) 43 (43) 5796–5799.
- L.E. Euliss, J.A. DuPont, S. Gratton, J. et al, Imparting size, shape, and composition control of materials for nanomedicine, Chem. Soc. Rev. (2006) 35 (11) 1095–1104.
- William R. Hendee, Kevin Cleary, Richard Ehman, et al; Bioengineering and Imaging Research Opportunities Workshop V: A Summary (2008) Annals of Biomedical Engineering, Vol. 36, No. 8,. 1315–1321.