Summary
The central methodological approach underlying this study was a pilot study designed to test the feasibility of using a translated survey questionnaire to identify patterns of interest among the sample. In particular, since the research question of the project was an attempt to determine the attitudes of Saudis regarding the relationship of good nutrition to colorectal cancer risk, the survey method was an integral part of the current study. However, as noted in the literature review, a gap was identified among the sources in the availability of a valid questionnaire scale to determine the patterns of interest. For this reason, it was necessary to develop our own scale or modify an existing scale in order to determine its validity, relevance, and relevance to the stated topic. The reason for not creating a proprietary scale was to save resources and time spent on this study. More specifically, the author’s position was that if a reliable, valid, and verified scale existed that had already been able to demonstrate performance on samples, then using it would solve the need for savings. Thus, it was decided to use an off-the-shelf questionnaire modified for the specifics of the current project.
A thorough search among academic sources for the given criteria led to an article by Smith et al. (2019), which evaluated the relationship between respondents’ eating habits and expected health outcomes. More specifically, the outcome of this study was to prove the validity of the DHCCBS questionnaire to produce results on assessing the relationship between variables. Since the DHCCBS questionnaire did show reliability and validity for measuring precisely the patterns that its developers intended, the present research project decided to use this material for a pilot test for a sample of Saudis. It is emphasized that the use of the specific questionnaire in the current study was implemented only with the full consent of the authors of the original article. Smith et al. were fully informed of the goals of the current project and the methodological steps that will be used to modify the original scale. Thus, only full informed consent justified the use of the DHCCBS for a future pilot trial.
Several key assumptions were made to ensure that this extrapolation would be valid. In particular, it is assumed that if the DHCCBS questionnaire were effective for measuring among U.S. respondents, it would also prove useful for a study aimed at measuring HBM patterns for Saudi audiences. In addition, it is assumed that translating this article into Arabic does not change the contextual meaning of the questions, thus preserving the validity of the survey questionnaire. It is also assumed that the sample for the current study is thoroughly familiar with the terms used in the questionnaire and has no difficulty interpreting them semantically. Finally, the primary assumption was that if the pilot study being initiated shows the expected results, then extrapolation to a larger sample, constructed according to the principles of representativeness of the Saudi Arabian general population, would retain validity.
Pilot Study
The design of this research project is based on the principle of a pilot test that will be able to confirm or refute the prospects of using the modified DHCCBS questionnaire for Saudi audiences. The pilot test was necessary because the potentially broad results of the study itself are of profound importance to the public health industry, and thus the academic responsibility for the published material is critically high. In addition, as stated, Saudi Arabia does not yet have a survey scale of its own, which means that the use of foreign material must be not only motivated but also scientifically sound. Thus, pilot testing on a small sample was a necessary step that would qualitatively improve the reliability of the DHCCBS extrapolation. In addition, because the use of the scale is a first for Saudi patients, pilot testing was a necessary step to verify the validity, especially in the public health industry, whose findings are critical to society.
It is essential to clarify that the pilot test cannot be conducted to test the stated hypotheses and find answers to research questions since this is not the intended mission of this approach. Nevertheless, the pilot test will identify fundamental limitations and errors that should be neutralized in the further stages of the research project development. Subsequently, these assumptions and corrections will improve the quality of the scale used for a broader study, first for a Saudi audience and then, if necessary, for a broader sample, such as the Gulf regions.
Reliability of Translation
In research projects based on the use of foreign materials, it is crucial to achieve reliability in which the results of this linguistic combination are valid and reproducible. Translating materials from English into Arabic requires special attention, thus, since negligence or ignoring contextual semantics poses threats to the quality of the entire study. At best, the loss of nuance can lead to ignoring minor details or increasing the time to complete the questionnaires because of the need for additional awareness of the logic of the questions. On the contrary, the worst-case scenario poses the danger of wholly misinterpreted questions and, as a consequence, poor-quality conclusions, which is particularly sensitive for clinical trials on promising cancer issues. Thus, situations in which a large-scale study using an inaccurately translated questionnaire would show that (for example) Saudis are unaware of the association of a proper diet with the development of colorectal cancer, although this is not true, should be excluded. This is why enough attention should be given to the procedure of translating the questionnaire material from English into Arabic to ensure that the copy is semantically identical. The methodological framework used for the correct translation procedure was selected from the research paper by Tsang et al. (2017) on the correctness of using translated questionnaires for clinical trials: the general process is demonstrated step-by-step in Figure 1 below.
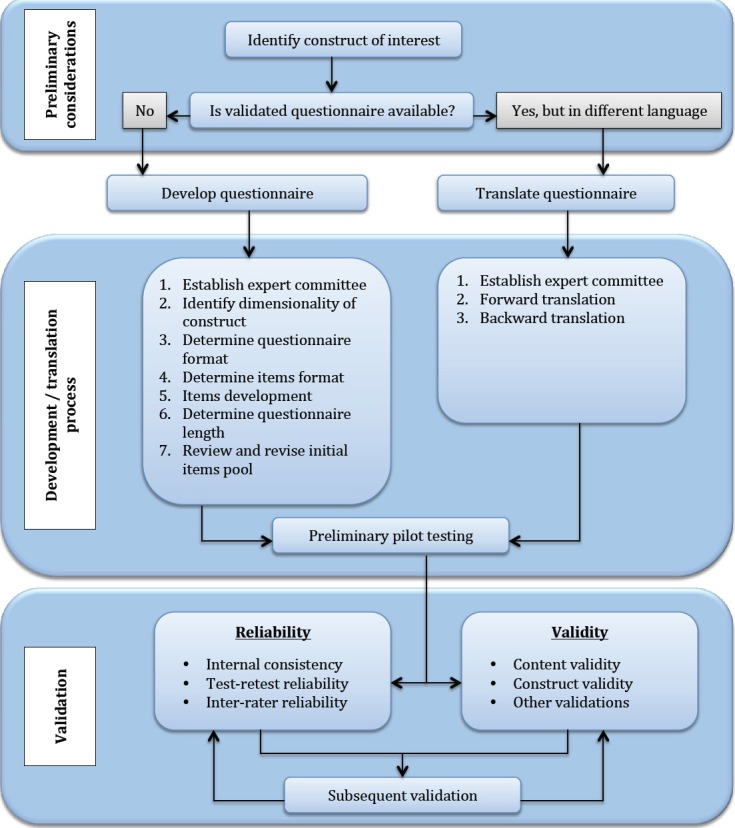
As can be seen from Figure 1, the survey translation procedure, once approved for use, should be conducted using a double translation technique. In this case, the source material in English is first translated into Arabic, and then the reverse translation is done. The need for back-translation is motivated by the need to verify consistency and preserve the contextual details of all questions (Shakhnovich, 2018). In the use of translation, as reported by Tsang et al. (2017), it is not enough to only translate survey material into Arabic even while preserving the complete contextual accuracy of the questions. Instead, the mechanics of the questionnaire need to be adapted to the specific objectives of the current study. In particular, since the questionnaire is designed to reveal — not in the pilot study, but in the following stages — Saudis’ awareness of the relationship between good nutrition and the development of colorectal cancer, it is highly likely that respondents will tend to misrepresent their answers in order to demonstrate their professionalism, even if this is not the absolute truth. Consequently, the format of the translated survey questionnaire should consider the potential for these distortions and try to minimize them.
Forward Translation
The direct translation procedure from English to Arabic must be carried out by at least two independent linguists whose proficiency in both languages is either native or close to the native speaker level. This strategy ensures diversity of opinion and reduces the likelihood of systematic error, while the requirement for independence of the translators responds to the threat of interaction between them, which would introduce an element of bias into the study. In addition, an additional requirement for this pilot test is the need for professional qualifications of an experienced physician, preferably in the oncology field. This requirement is related to the use of specific medical terms that may not be understood by the interpreter, which would affect the quality of the survey material being broadcast. Thus, two bilinguals were chosen for the present test, including a medical oncologist and an information technology student. The motivation for selecting this faculty student stems from the assumption that information analysis skills would be useful in translating the survey material. Additionally, according to Tsang et al. (2017), one of the independent translators had to be unfamiliar with the concepts of the target survey to allow for more accurate control of subtle differences.
Therefore, each translator received a survey document in the original English language and was asked to translate this material into their native Arabic, using the most accurate constructions possible, preserving the semantic identity of the texts. Based on the results of the direct translation, the researcher had two independent surveys for which a synthesis had to be found. For this purpose, a third, unbiased bilingual, who has a medical degree, was invited to collaborate with the author of the project to form a single questionnaire document based on the two created translations, which would meet the criteria of semantic accuracy, contextual closeness, grammatical accuracy and logical presentation of the questions as much as possible.
Reverse Translation
A back-translation procedure for a newly created survey material from Arabic to English would allow any hidden inaccuracies to be detected and the translation to be further edited if necessary. Ensuring maximum independence to achieve translation validity was realized through the use of additional two translators who had English as their first language. For this purpose, it was decided to turn to local expats from the United States who were excellent not only in English but also in Arabic, and therefore could assist in this study. Interestingly, Saudi Arabia is an excellent environment for an expatriate approach, as the Vision 2030 programs and government reforms of Crown Prince Mohammed bin Salman Al Saud have been reasons for the increased investment popularity, including through foreign labor, of Saudi Arabia in the global capital market (Lazell, 2021). Moreover, the choice of expats as independent translators was further motivated by the fact that their expertise did not include knowledge of oncological disciplines, and therefore the questionnaire created in Arabic could be translated into English with a minimum of bias. Thus, the twice-translated DHCCBS questionnaire would be compared with the original material. Any deviations, differences, and inaccuracies relevant to differences in semantic perception will be corrected if necessary.
Expert Commission
The translation of the survey material, back-translation, and corrections, if needed, should be qualitatively checked with the help of an expert committee. This strategy allowed for the creation of a pre-final questionnaire file that accurately reflects the desired context and meets the stated criteria to achieve validity. The expert panel should include the author of the current project, the methodologists, the translators of the two tracks, and if possible, the developers of the original survey material, which are Smith et al. The panel will have on its agenda a qualitative comparison of the resulting material and the original DHCCBS; it is critical that full understanding of all issues be reached. Any discrepancies and inaccuracies are handled collectively. An additional statistical check of the translated material using Cronbach’s Alpha degree of consistency is conducted at this stage. Cronbach’s Alpha is a psychometric parameter that assesses the reliability of consistency between different parts of the questionnaire (Taber, 2018). In this way, it is possible to construct a pre-final questionnaire that can be used seamlessly for pilot testing.
Study Design
The methodological basis for this study is based on a mixed-methods approach, using both qualitative and quantitative techniques to elicit responses. If the pilot test shows high validity using the DHCCBS, subsequent phases should focus on evaluating research hypotheses and seeking answers to the research questions posed. Thus, post-pilot studies are expected to seek both qualitative and quantitative answers. From a qualitative perspective, the answers will shed light on the potential relationship between perceptions of healthy eating and patterns associated with the development of colorectal cancer. From a quantitative methodology, DHCCBS will assess numerical trends relevant to the Saudi audience: the dynamics of responses, the correlation between them, and the chi-squared distribution of shares. The use of a quantitative approach is driven by the mechanics of the survey, in which the questions are structured along the lines of a psychometric Likert scale. Specifically, this DHCCBS design included thirteen questions assessing different aspects of HBM in sequence. Among other things, this would provide conclusions for cohort testing, in which perceptions of the association between proper diet and colorectal cancer risk are tested separately by gender, age, and occupational criteria. Taken together, both qualitative and quantitative trial approaches will provide an overall picture that reflects the research question’s agenda. Meanwhile, the experiment design is based on a cross-sectional measurement of trends, which means specific patterns were measured for audiences in the same time period (Cherry, 2019). This limited the ability to examine causal relationships in determining the nature of the relationship, but this was not an issue for the current research project.
Creating a Sample
There are important limiting criteria for a pilot test that do not allow the project to be scaled up. In a general sense, the pilot test should not be large-scale because the goal is not to use a representative sample to produce results on a research question. Therefore, with limited budget and needs, it is recommended to use a sample of no more than thirty participants. The motivation for this number is justified by the minimum sample size requirements in psychometric and social tests: a vice of thirty respondents is precisely the minimum required (Gause, 2018). A pilot test will be conducted for thirty respondents, the purpose of which is to test whether the translated DHCCBS can be used for the purposes of broader research in subsequent phases.
Sampling among Saudi residents will be based on the use of social media contacts and personal acquaintances. This method significantly simplifies the technical part of the study and saves resources (Joseph et al., 2016). Nevertheless, this approach must ensure that systematic sampling error is minimized, even though it is used as the respondent group for the pilot test. More specifically, participants from all regions of Saudi Arabia are invited for the pilot phase, as if it were the sample for the present study. This strategy helps to cover the needs for minimal representativeness of the pilot sample and the detection of potentially hidden problems that may be identified by regional residents but not detected by respondents from Riyadh.
Pilot sample participants are collected through the mailing of an invitation link to participate. The researcher’s tasks at this stage include critical monitoring of the demographic and geographic metrics of the invited individuals to cover the goals of the stated minimum representativeness. Along with the link to the survey document located on the Google Drive cloud storage, participants receive informed consent, which introduces them to the goals of the testing being conducted, discloses sensitivities, and asks for a personal signature as a sign of full, informed consent to participate; one informed consent must be collected from each participant in the pilot sample, without which participation in the survey is impossible.
Participants completed the questionnaire in one of two ways, depending on personal desire. The first option involved printing the file, filling it out by hand with a pen, scanning it, and sending it to the researcher via the appropriate field. This was a convenient option for older respondents who had trouble reading information from a computer screen. It also made sense for those participants in the pilot sample who did not have a computer. The second participation option was based on completing the online survey via computer, which is less resource-intensive and saves time. Choosing either option to complete the DHCCBS questionnaire does not affect the subsequent processing of the results.
Results Processing
The question of the validity of using the modified DHCCBS is answered by using quantitative methods to evaluate the results obtained. Specifically, this includes determining the mean, SD, and IQR to judge trends common among the pilot sample. In addition, content analysis is proposed to determine the most frequent verbal constructions reflecting the responses of the sample. Additionally, the use of Cronbach’s Alpha consistency criterion, Pearson’s coefficient to test the reliability of repeated trials and to determine the validity period of the questionnaire is suggested (Tsang et al., 2017). All of the tools used together allow us to not only assess the questionnaire’s applicability to broader studies but also to simulate results that may be obtained in future questionnaires on larger samples.
Ethical Aspects
The study will adhere to the ethical principles of the Research Ethical Committee from the Fakeeh college of medical sciences. This study is built on the principle of academic integrity, which means that any material used is carefully cited and not copied or plagiarized in any way. The full-text documents of the research papers have been obtained legally through digital library databases. In addition, the author has no interest in distorting the results or selecting the final data in such a way as to satisfy any particular outcome of the study: any results have value. All participants in the pilot trial were familiar with the procedures of the entire experiment and signed informed consent.
References
Cherry, K. (2019). How does the cross-sectional research method work? VeryWell Mind. Web.
Gause, T. (2018). Why is sample size n>=30 sufficient? Isixsigma. Web.
Joseph, R. P., Keller, C., & Ainsworth, B. E. (2016). Recruiting participants into pilot trials: Techniques for researchers with shoestring budgets. Californian Journal of Health Promotion, 14(2), 81-89.
Lazell, M. (2021). An introduction to Saudi Arabia. Expatica. Web.
Shakhnovich, V. (2018). It’s time to reverse our thinking: the reverse translation research paradigm. Clinical and Translational Science, 11(2), 98-99. Web.
Smith, K.S., Raney, S.V., Greene, M.W. & Frugé, A.D. (2019). Development and validation of the dietary habits and colon cancer beliefs survey (DHCCBS): An instrument assessing health beliefs related to red meat and green leafy vegetable consumption. Journal of Oncology, 1-7. Web.
Taber, K. S. (2018). The use of Cronbach’s alpha when developing and reporting research instruments in science education. Research in Science Education, 48(6), 1273-1296. Web.
Tsang, S., Royse, C. F., & Terkawi, A. S. (2017). Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi Journal of Anaesthesia, 11(1), 80-89. Web.